Statement of the ACNFP on Precision Bred Organisms - July 2023
Statement of the ACNFP on Precision Bred Organisms - July 2023
In this guide
In this guideExecutive Summary
The FSA has the mandate to assure all food on the market is safe and it is what it says it is. To support and inform the FSA’s policy development in the area, the Advisory Committee on Novel Foods and Processes (ACNFP) was tasked with considering the scientific basis of the technologies used in precision breeding. This included providing scientific advice relating to the types of data that could be used in the safety evaluation of Precision Bred Organisms (PBOs) for use as food and feed. An expert Subcommittee on the Products of Genetic Technologies (PGT) was established to assist the ACNFP with this work in anticipation of the need for new technical guidance.
An organism is determined to be a PBO by Defra’s Advisory Committee on Releases to the Environment (ACRE) if the changes introduced by modern biotechnology are considered to be equivalent to those that could have been produced through traditional breeding methods (TB). Full technical definitions of PBO and TB are available in the Genetic Technology (Precision Breeding) Act 2023. In September 2021, the ACNFP was commissioned to advise on the science that could be applied in a tiered approach to the safety assessment of PBOs and the determination of criteria to be used to assign organisms to these tiers (FSA Board meeting papers, September 2021).
The first two statements of advice can be found on the FSA ACNFP website. These outline the basis for the ACNFP’s agreement that a two-tier assessment process for PBOs allows a proportionate and scientifically justifiable level of scrutiny. Triage questions were also developed focussing on novelty, composition (covering aspects of nutrition, toxicity, allergenicity), and other safety concerns (on a case-by-case basis) to determine Tier assignment. Tier 1 PBOs are those for which the answers to the triage questions provide sufficient information to determine that no further review is required. Where answers to the triage questions identify the need for further specific scrutiny, these PBOs would be assessed in Tier 2.
This statement addresses the third phase of work commissioned by the FSA: namely the determination of what information (data requirements) should be requested from applicants to support the safety assessment of a PBO for food and feed.
The ability to assess the risk (if any) to consumers and animals from the consumption of PBOs and products of PBOs in food and feed, requires information and evidence on the nature (and novelty) of the product, on aspects of expected use/exposure, and understanding any potential hazard. The interpretation and integration of this information into effective scientific advice for policy making should be proportionate to the extent and nature of any risk identified.
All foods marketed in the UK need to comply with General Food Law (GFL) and this will also be true for PBOs no matter the approach taken to their assessment and regulation. Over many decades, due diligence within industry has been accepted by the regulator as adequate for managing potential safety risks of traditionally bred organisms (TBO)s. This reflects the fact that food and feed safety concerns identified in TBOs have been few, and managed effectively within GFL. However, for PBOs, the ACNFP agrees that a two-tier risk assessment approach is diligent and proportionate for assessing organisms developed using this emerging technology.
To inform the development of data requirements, the ACNFP and its PGT subcommittee discussed and acknowledged the need for proportionality as required by the Genetic Technology (Precision Breeding) Act 2023 and the FSA board principles for the development of policy on PBOs (September 2021). In an attempt to determine the potential hazards that could be posed by PBOs, the ACNFP has considered both what is understood scientifically about PBOs and what remains unknown about this rapidly evolving technology and how it may be applied in future.
Two workable Models (see Figure 2 in the main paper) have been developed for evidence-based safety assessment, either of which could in principle be implemented. The preferred approach of the FSA for risk management will be chosen taking account of the level of scrutiny and safety assurance considered necessary for PBOs. These Models and the types of data that could be required in each are summarised briefly:
- Model 1 focuses on the equivalence between PBOs and TBOs, and on the genetic change and its intended phenotype. The data requirement for safety assessment is predominantly descriptive and confirmatory, with details of the change(s) provided and the description of the resulting product. Compositional data is typically not required in the initial submission. Quantitative data on phenotype is required but mainly focuses on verifying that the intended trait, if relevant to food or feed safety, has been achieved.
- Model 2 builds on Model 1 but focuses on the wider phenotypic consequences of precision breeding and the impact of these on the PBO as consumed. It requires a broader suite of compositional data to be submitted in the initial application. This reflects the view that the new nature of the technology justifies a level of additional scrutiny. Additional to the Model 1 data requirements, compositional data (nutrients and anti-nutrients, metabolite information, proximate analysis or alternative approach (for plants), and edible-by-products data (for animals)) would be routinely required as part of the submission of proposals to inform the considerations of any inherent potential for toxicity and / or allergenicity.
In both Models, the safety assessment is conducted in a tiered or structured approach after answering two questions:
- Question A: Does the PBO have a history of consumption as a food or feed? And
- Question B: Are there any concerns regarding nutritional disadvantage, toxicity or allergenicity?
Tier 1 PBOs are those where sufficient information is provided in an initial data submission to complete a safety assessment satisfactorily. If that is not the case, a more detailed safety assessment in Tier 2 is initiated. The nature of any additional data required will be determined on a case-by-case basis, dependent on the organism and any potential hazards identified.
The way PBOs are intended to be managed impacts the initial data requirements that support Tier assignment in each Model. Both Models use a risk- and evidence-based approach to PBO assessment, based on novelty and anticipated concerns. These Models offer two distinct data requirement options to the FSA. Which Model is preferred will depend not just on the level of safety scrutiny and assurance offered by each approach, but also on wider considerations of risk management and policy.
The technical justification, depth of assessment, and further discussion on the strengths and weaknesses of each model option are discussed within this statement. This paper provides context for the models developed and is intended to support further discussion by the FSA on an approach that meets its policy goals.
Abbreviations
In this guide
In this guide|
Acronym |
Definition |
|
ACNFP |
Advisory Committee on Novel Foods and Processes |
|
ACNFP-PGT |
ACNFP Products of Genetic Technologies Subcommittee |
|
ACRE |
Advisory Committee on Releases to the Environment |
|
CRISPR/Cas9 |
Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9 |
|
Defra |
Department for Environment, Food and Rural Affairs |
|
DHSC |
Department of Health and Social Care |
|
FAO |
Food and Agriculture Organization of the United Nations |
|
FSA |
Food Standards Agency |
|
GFL |
General Food Law |
|
GMO |
Genetically Modified Organism |
|
NAMs |
New Approach Methods |
|
NF |
Novel Foods |
|
PB |
Precision Breeding |
|
PBO |
Precision Bred Organism |
|
PGT |
Products of Genetic Technologies |
|
SoS |
Secretary of State |
|
TALEN |
Transcription Activator-Like Effector Nuclease |
|
TB |
Traditional Breeding |
|
TBO |
Traditionally Bred Organism |
|
UK |
United Kingdom |
|
WHO |
World Health Organisation |
1. Introduction
In this guide
In this guide1. The UK Advisory Committee on Novel Foods and Processes (ACNFP) advises the Food Standards Agency (FSA) on matters relating to the safety of products of modern biotechnology destined for food and feed purposes, including products from Genetically Modified Organisms (GMOs) and Precision Bred Organisms (PBOs). The ACNFP provides assurance through evidence and risk-based assessment of food and feed innovation, that food and feed on the market:
- is safe to eat
- does not mislead the consumer
- does not put consumers at a nutritional disadvantage
An expert Subcommittee on the Products of Genetic Technologies (PGT) was established to assist the ACNFP with this work.
2. As described in the Genetic Technology (Precision Breeding) Act 2023, organisms (and the food and feed derived from them) produced by modern biotechnology techniques, such as genome editing, that could also have been produced through traditional breeding (TB) processes, will be classified by Defra as PBOs and will no longer fall under the scope of the Genetically Modified Organisms (Deliberate Release) Regulations 2002. The scope of the Act covers both precision-bred plants and animals. The decision whether a product of modern biotechnology is a PBO or a GMO lies with the Defra Secretary of State (SoS), following the receipt of a report from the UK Advisory Committee on Releases to the Environment (ACRE). Further detail on this process will be released by Defra.
3. Ministers have been granted powers in that Act to make regulations that will allow the FSA to establish a regulatory framework for the safety assessment of PBOs used in food and feed. The FSA will consider how to assess the safety of organisms designated as PBOs for food and feed uses, in a proportionate and effective manner to offer assurance of consumer safety. A recommendation that takes account of a range of factors will be made by the FSA for final decision by the DHSC SoS.
4. In addition to the scientific uncertainty that is present in all safety assessments, it is noted that the technology involved in the generation of PBOs is rapidly evolving and any process and guidance needs to be future proofed for the coming years, as well as satisfying the needs of today. This is reflected in the advice of the ACNFP on the approach to the assessment of PBOs that was detailed in the statements from the Committee published in September 2022 ACNFP statement and January 2023 ACNFP statement.
5. To support the development of a regulatory approach to safety assessment, the ACNFP (as supported by the work of the PGT Subcommittee) reviewed a number of different case studies detailed in Annex A (plants and animals) to gain insights into current scientific understanding of the safety of food and feed produced by technologies used in precision breeding (PB). In developing its advice, the ACNFP discussed the scientific and technical principles that could be used to underpin the data requirements for operating a proportionate and effective regulatory framework, thereby meeting the policy commission.
6. The ACNFP, through the review of case studies, has seen no evidence that PBOs are intrinsically more hazardous than traditionally bred organisms (TBOs). It was noted that in terms of genetic changes, any TB technique is likely to introduce a greater number of new genome variants than that obtained through technologies used to produce a PBO. It is recognised that a range of phenotypic outcomes is possible from both TB and PB, although these may be more easily achieved with PB technologies. It is this impact on phenotype that the triage questions seek to understand.
7. The ACNFP concluded in its first statement that, as with any breeding process, use of PB technologies has the potential to create safety risks for consumers and these need to be identified, assessed, and managed appropriately and proportionately. A two-tiered assessment process for PBOs was therefore proposed by the FSA, to provide clarity for applicants while allowing appropriate scrutiny of the possible risks as part of the assessment process.
8. As further detailed in ACNFP statements 1 and 2, the definition of Tier 1 and 2 as defined in the FSA September 2021 board paper are:
- “Tier 1: All applications for PB food and feed authorisations are screened for similarity to traditionally bred varieties where the risk is understood and not of concern for consumers. Organisms that meet Tier 1 criteria will be authorised more quickly than Tier 2. The detailed criteria for assessing Tier 1 applications are still being developed, informed by expert scientific advice from the independent Advisory Committee on Novel Foods and Processes (ACNFP).”
- “Tier 2: Applications for PB food and feed authorisations where the Tier 1 screening does not allow the risk to be understood are subject to an additional step. These applications require a proportionate risk assessment to determine the level of risk for consumers”.
9. An overview of the safety assessment process with two tiers was provided by ACNFP in the ACNFP's second statement. Following notification of a PBO from ACRE, a series of triage questions, focussing on novelty, composition (toxicity, nutrition and allergenicity), and other safety concerns, can be used to guide assignment to Tiers. Tier 1 PBOs are those for which the answers to the triage questions provide sufficient information to determine that no further review is required. Where answers to the triage questions identify the need for further specific scrutiny, these PBOs would be assessed in Tier 2. Tier 2 allows further scrutiny and requests for further data to be generated if concerns are identified and there is potential for increased risk to consumers. The justification for further data must be explained. The criteria and associated triage questions are listed in Table 1.
Table 1. Criteria and associated triage questions to support the assignment of PBOs to Tier 1 or 2
(ACNFP second statement)
|
Criteria |
Associated triage question |
|
Novelty |
Is the PBO from a species that has no significant prior history of safe consumption in the UK or EU? |
|
Composition – Nutrition |
Is the PBO designed to introduce significant changes to the nutritional quality of the organism currently consumed that are likely to be disadvantageous to the consumer? |
|
Composition – Toxicity |
Is the PBO designed to introduce changes that are expected to elevate significantly the toxicity of any foods/feeds derived from the organism? |
|
Composition – Allergenicity |
Does the PB introduce changes that are expected to alter the allergenicity of any foods/feeds derived from the organism? |
|
Other safety concerns |
Are there any additional features of the PBO that cause food/feed safety concerns? |
10. Whilst there is no evidence that the current system of due diligence is ineffective for TBOs, it is noted that the scientific logic underpinning the framework and data requirements for PBOs could also be applied to TBOs meeting similar criteria. In the case of PBOs, in the early years of adoption of these new technologies, it should be reassuring to consumers that the innovative nature of the methods involved in PBO production are being carefully considered by producers as part of a regulatory process.
11. In developing the possible PBO specific data requirements, the ACNFP was mindful of the wider policy context in which it operates. Within the Genetic Technology (Precision Breeding) Act 2023 there is a requirement that the assessment of safety is proportionate. The Committee noted the potential for different interpretations of proportionality and therefore the level of assurance required from the assessment. This has informed the development of two model options the FSA could adopt with different initial data requirements to address these differing interpretations. One focuses on the technical equivalence of PBOs and TBOs and the other focuses on the uncertainties and unknowns around how the rapidly evolving PB technology could be used in the future to develop organisms with intentionally designed traits for food and/or feed use.
12. This statement summarises the initial data that could be required in the two model options, in order to review PBOs for potential food and feed safety risks. It also outlines the information needed for both Tier 1 and Tier 2 assessments.
13. Both Models 1 and 2 use a risk- and evidence-based approach to tiered assignment of PBOs. The Models’ requirements provide transparency on what may be necessary to provide assurance of the safety of PBOs for food or feed.
14. The depth of assessment, and further discussion on the strengths and weaknesses for each model are discussed within the statement. This, along with the technical justification for data provisions in Annex B, provides context for the potential data requirements that are outlined.
15. It is noted that risk managers, in making their decision on the level of data required to provide adequate assurance of the safety of PBOs, will be taking account of a range of other factors in addition to safety-relevant data. These include burdens on industry, public attitudes, the possibility that more in-depth review might unnecessarily heighten safety concerns about PBOs, barriers to innovation and the potential benefits of this new technology. Consideration of each of these other legitimate factors may make one or other of the proposed data model options more or less preferred.
16. There is provision in the Act for consideration of other legitimate factors in overall decision-making by the Secretary of State (SoS) in authorising PBOs. These could include, for example, impacts on animal welfare. However, since this was beyond the remit of the ACNFP, other factors were not considered. While animal PBOs are expected to be subject to additional legislation including consideration of animal welfare, the ACNFP advice on data requirements has been developed so that it can apply to both animal and plant PBOs as food and feed when applications are received.
2. Committee outcome on the data requirements for Tier 1 and Tier 2 assessment in the context of two models
In this guide
In this guide2.1 Proportionality and the options for FSA approaches to data requirements
17. The Genetic Technology (Precision Breeding) Act 2023 confers on the FSA the responsibility to ensure that the regulatory approach to safety for food and feed produced by PB techniques considers “proportionality” as one of the five key underpinning principles of the PB regulation design. The regulatory framework needs to be developed to allow safety assessment of anticipated and specific safety issues associated with PBOs used for food or feed but at the same time support innovation to allow the potential benefits to be realised in a safe and sustainable way. There is therefore a need to consider the minimum data required to ensure that a risk-based safety decision can be made.
18. The ACNFP previously noted that the Act can be interpreted as making an implicit equivalence claim, namely, that TBOs and PBOs have similar risk profiles. This is because PBOs are defined in the Act as organisms produced by modern biotechnology that “could have been produced through traditional breeding processes”. ACNFP Members recognised that most organisms produced by PB will be similar in risk profile to their traditionally bred counterparts, where a safety assessment is not required. However, some organisms produced by TB may also have risks, such as modification of antinutritional factors or alteration of the allergenic potential. These risks are currently managed under due diligence requirements.
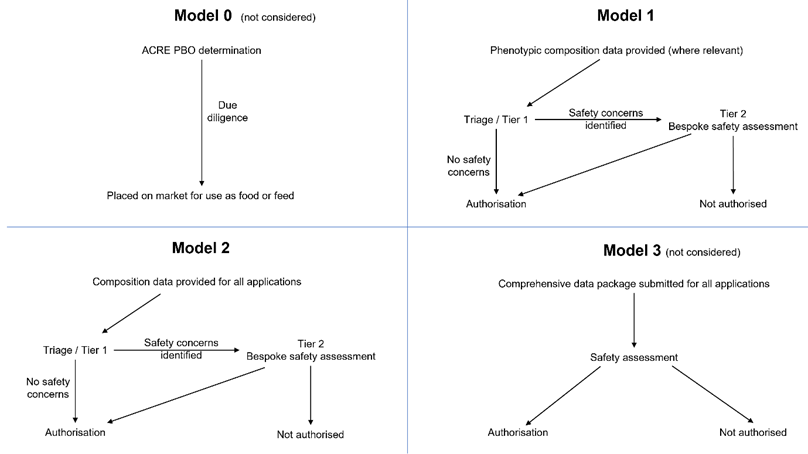
19. Although two scientifically valid models of triage are described in this statements, four models of data requirements could be foreseen (Table 2). ACNFP notes that Model 0 remains a policy option. It would involve no pre-market safety assessment and thus no data requirement for PBOs. However, it was not considered by the ACNFP as its remit was to consider the data requirements for proportionate pre-market safety assessment.
20. A fourth model, Model 3 (Table 2) was briefly discussed by the ACNFP. This would represent an approach to safety evaluation that could provide greater assurance than Model 1 or 2, but it would call for a large battery of compositional and toxicological tests similar to those required for a novel food product. However, this was considered to be excessive for most PBOs. Hence, the focus was given to illustrating data requirements for Models 1 and 2. A schematic representation of the four models can be found in Figure 1.
Table 2. Different models of the approach to triage for the assignment to Tier 1 or Tier 2 during assessment of PBOs
ACNFP has developed both Model 1 and Model 2
|
Triage approaches |
Reasoning of the approach, and extent of the data requirement for triage |
Comment |
|
Model 0 |
PBOs are of equivalent risk to TBOs. (PBOs and TBOs have equivalent safety profile). - No data reviewed, subject to due diligence under the General Food Law. |
For reference only – not considered due to no pre-market safety assessment or data requirement. |
|
Model 1 |
PBOs are equivalent to TBOs, but whether the intended trait is likely to affect food/feed safety needs to be understood. - Information requirement builds on due diligence and is minimal - may include compositional data, but these will not go beyond that required to verify that the intended phenotype has been achieved (for deliberate changes in composition relevant to the quality of food/ feed). The comparator in this model is a TBO with the same or very similar genetic change and phenotypic trait. |
Model identified as scientifically valid – and fulfils policy commission. |
|
Model 2 |
PBOs are equivalent to TBOs, but the innovative nature of the technology used justifies a higher level of scrutiny than Model1, requiring additional compositional data to give a higher level of assurance. - Model 2 builds on the data required to support due diligence and Model 1. In addition, routine data requirements include additional compositional (nutrients/anti-nutrients, toxicology, allergenicity) data. The comparator for the compositional criteria in this model is the organism prior to genetic change. |
Model identified as scientifically valid – and fulfils policy commission. |
|
Model 3 |
Exhaustive assessment of the PBO as a novel food. - Data requirement includes all of the above plus intensive higher tier toxicological or clinical studies (akin to those that might be required for a novel food under that current regulation). |
For reference only – Model identified as not justifiable and may not fulfil the policy commission. |
21. Models 1 and 2 (defined below) diverge on the level of compositional data to be provided in the initial submission to allow for triage into Tier 1 and Tier 2 assessments (defined above). They represent different intermediates in a scale ranging from minimal to more extensive data requirements (Table 2). Data that are considered ‘necessary’ will depend on the level of uncertainty risk managers are content to accept in the safety assessment, and the interpretation of proportionality that policymakers wish to apply when balancing safety assurance and other legitimate factors judged to be within their remit.
- Model 1 focuses on the equivalence between PBOs and TBOs, and on the genetic change and its intended phenotype. The data requirement for safety assessment is predominantly descriptive and confirmatory, with details of the change(s) provided and the description of the resulting product. Compositional data is typically not required in the initial submission. Quantitative data on phenotype is required but mainly focuses on verifying that the intended trait, if relevant to food or feed safety, has been achieved.
- Model 2 builds on Model 1 but focuses on the wider phenotypic consequences of precision breeding and the impact of these on the PBO as consumed. It requires a broader suite of compositional data to be submitted in the initial application. This reflects the view that the new nature of the technology justifies a level of additional scrutiny. Additional to the Model 1 data requirements, compositional data (nutrients and anti-nutrients, metabolite information, proximate analysis (for plants), and edible-by-products data (for animals)) would be routinely required as part of the submission of proposals to inform considerations of any inherent potential for toxicity and / or allergenicity.
Figure 1. Schematic representation of four possible models.
Nature of the data requirements for each, and route between Tiers for the safety assessment of PBOs.
2.2 Data requirements
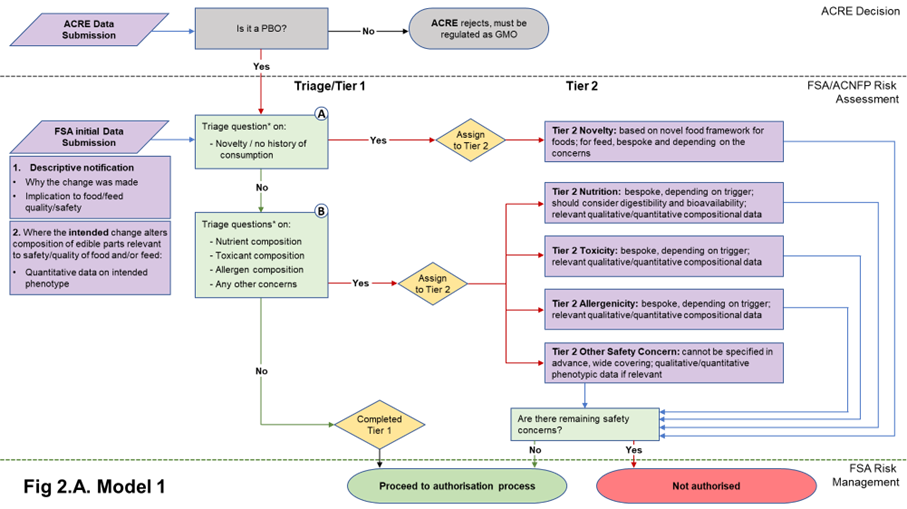
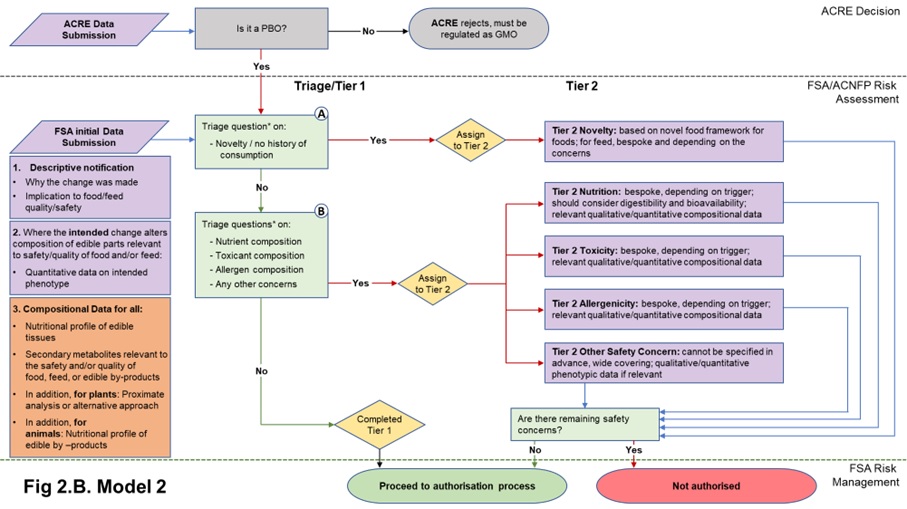
22. The data requirements in both Models 1 and 2 start with a core set of information needed to understand the PBO for which the applicant is seeking authorisation. Model 1 includes a basic composition requirement in the form of quantitative data on the phenotype, to assure the assessor that the PBO is what it says it is. Model 2 also requires this information, plus additional compositional (nutrients, anti-nutrients, metabolites, proximate analysis of edible tissues (plants); nutrients, metabolites of edible tissues and edible by-products (animals)) analysis. The data submitted in the initial submission in either case will be used to answer the triage questions described in Table 1 and determine Tier status. The nature of the compositional data required depends on the model adopted. Flow diagrams representing the two Models and their data requirements are presented in Figures 2A and 2B.
23. In developing the data requirements, the potential risks that might occur were based on the case studies reviewed by the ACNFP (Annex A). This review resulted in a detailed set of technical justifications of the types of data that might be needed in different circumstances. These are explained in detail in Annex B and form the underpinning for the data requirements identified.
Figure 2. Flow diagrams for the regulatory assessment of PBOs.
Figure 2. (A) In Model 1, the focus is the nature of the genetic change and its immediate phenotypic consequences. (B) In Model 2, the focus is on the wider consequences of the genetic change. PBO status is determined by ACRE; FSA / ACNFP conduct safety assessment to advise on safety for authorisation for use as food/feed. In addition to descriptive information (1) and compositional data on the trait (2) required in Model 1, Model 2 also requires additional compositional and nutritional data (3). When safety concerns are identified during steps A and B of the assessment (triage questions* listed in Table 1), applications are assigned to Tier 2, where data requirements are on a case-by-case basis. Purple/orange: data submission; green: data assessment; yellow: outcome of triage.
2.2.1 Non-compositional descriptions of all PBOs in the initial data submission
24. A non-compositional initial submission is required of all applications and would be the same for both Model 1 and Model 2. This will consist of a description of the identity of the PBO and its characteristics (i.e., how it compares to its traditionally bred counterpart, and the hazards or risks it may present) to allow triage. This information builds on what is expected to be required for submission to ACRE for determination of PBO status but should be tailored for suitability to food and feed safety assessment.
25. In order to understand the genomic change made to create the PBO, the applicant should provide information on:
- The target gene(s), i.e., name(s) and primary function(s) together with the reason for targeting.
- Description of the impact of the genetic change and how it achieves its mode of action.
- The intention or purpose for making the change.
- The materials and methods used to obtain the alteration, including evidence that any edit was made where intended, and a description of the analysis or procedures undertaken to minimise the potential for unintended alteration of the organism’s genetic material (so-called “off-targets”), and confirmation of the absence of relevant vector-derived sequences.
- Description of the predictable effects of the genetic change on genomic features at the site of insertion of a cisgene.
26. To further support the understanding of the PBO as a food or feed, the following information should be provided:
- Identification of the parts destined for food and/or feed use, and;
- Intended use, for example, food and/or feed; the animal(s) for which a feed would be intended should be identified. Information on the contribution of the food to the overall diet of the population and information on likely processing before use for food and/or feed may be helpful.
27. To allow determination of the novelty of the PBO, based on the associated triage question (Table 1), applicants must identify whether the organism subject to the -precision breeding or products derived from it have a significant prior history of safe consumption in the UK or EU. The applicant should provide:
- Taxonomic information on the organism (Family, Genus, Species) and a statement on the history of safe use of the PBO species relating to food and/or feed use.
28. In addition to the information identified in the data requirements, some information was flagged as providing helpful context, if available to applicants. Although all organisms without a history of consumption (novelty, as defined above) would require Tier 2 assessment, not all new traits introduced to a species would require further scrutiny in Tier 2. A trait new to a PBO may have a history of safe use from a closely related species with a similar role in the diet. Information which could be provided to support this may include, but not limited to:
- Information on homologous genes in closely related organisms (where the function of an introduced cisgene is novel to the host species), and;
- Information on food and/or feed products or organisms already on the market containing an equivalent trait or mutation in homolog gene(s), and with the same function in the diet.
29. It is recommended that applicants be asked to provide comprehensive information in a statement reviewing how the introduced trait could impact the quality of food and/or feed. They should also offer their scientific evaluation and conclusion in considering how nutritional quality and safety profile may be significantly altered.
30. The technical justifications presented in Annex B highlight particular areas of concern for the safety of PBOs for food and/or feed, which may support the applicants in identifying information that would be relevant to submit. The type of information provided could include, but not limited to:
- Where loss, gain, or change of function of the endogenous DNA and/or other “on-target” impacts in addition to the precise intended edit were identified at the site of the genetic change, evaluation of the likely impact relevant to the quality of food and/or feed, based on the available knowledge. For animal PBOs, when limited information is available on the function of the endogenous DNA, information on the function of homologs in other organisms.
- Description of the intended trait.
- Description of predicted changes in physiology of the plant or animal.
- Description of metabolic or regulatory pathway(s) with which the genetic change may be anticipated to interfere, including the resulting potential impact on the quality of food and/or feed, based on the available genetic and physiological knowledge; for animal PBOs, where functional genetics is less advanced, knowledge of the function of homologs in other organisms may be relevant.
- Information on any likely significant alteration in protein expression and/or change in its allergenic potential.
- Confirmation that levels of antinutrients, toxins or allergens known to the host species are not expected to be affected by the change.
2.2.2 Compositional data relevant to the quality of food and/or feed in initial data submission in Model 1
31. By principle, the Model 1 approach to triage and Tier 1 assessment focusses on the genetic change and its intended phenotype. Some phenotypic changes introduced into a PBO may not be relevant to the quality of food and/or feed. Understanding such relevance will allow focussed scrutiny where necessary and avoid analysis of information irrelevant for food safety. It was noted that information or data to support this should already be available to the applicants and should have been used to underpin their reasoning when ensuring due diligence to comply with GFL.
32. Where the intention of the PB is to intentionally change the composition in the PBO in a way that is likely to affect the quality of food and/or feed, the applicant should provide:
- Compositional data to demonstrate that the desired phenotypic change has been achieved. This is both to understand the significance of a phenotypic change relevant to the quality of food and/or feed, and to ensure that a PBO for food and/or feed “is what it says it is”. For Model 1, no further compositional data should be required as part of Tier 1.
2.2.3 Additional compositional data in Model 2 of triage in the initial data submission
33. The Model 2 approach to triage and Tier 1 assessment focusses on wider phenotypic consequences of the genetic change, as opposed to solely those which were intended. This requires understanding any compositional changes relevant to food and/or feed in the organism. Whilst requiring more data than Model 1, the aim remains to keep the additional data requirement to a minimum while providing a higher degree of assurance by comparison to Model 1. Other models are available that would provide further assurance but these were not considered in detail, as explained above.
34. Model 2 data requirements build on those of Model 1, i.e., data on deliberate compositional changes introduced by PB. In addition, further compositional data relevant to the context of the genetic change (i.e., depending on the host organism and what can be anticipated from the nature of the induced change) is required. This additional requirement differs for plants and animals, due to the wider range of phenotypes possible in plants, compared with animals.
- For plants: for the edible part(s), applicants should provide nutritional profile, proximate analysis (including vitamins, minerals for example) or alternative approach, levels of secondary metabolites relevant to the species or the anticipated changes, which are pertinent to nutrition, toxicology or allergenicity. This will provide a high-level compositional profile or specification for the organism.
- For animals: the focus would be on the composition of all derived edible products, not only the tissues of the PBO but also for example, milk or eggs. Nutrient profile, levels of secondary metabolites relevant to the species or to the anticipated changes which are pertinent to nutrition, toxicology or allergenicity would need to be provided for these derived food products.
35. Data submission should be tailored to the composition profile of the PBO and consider known hazards, to enable the questions relating to triage to be answered effectively, using appropriate reference databases.
2.2.4 Data requirements for Tier 2 assessment
36. Where the information provided in the initial data submission elicits a positive (‘yes’) answer to any of the triage questions (Table 1), or where there is insufficient information to understand the possible safety concerns presented by a PBO, further assessment will be triggered as part of a Tier 2 assessment.
37. Each triage question triggers a specific Tier 2 assessment: the data requirements for Tier 2 assessment will depend upon the factors which identified a need for further scrutiny, i.e., bespoke and considered on a case-by-case basis. Therefore, there cannot be a predetermined list of data requirements or tests for Tier 2. However, technical guidance will be developed for the applicants in terms of what should be provided in the initial assessment dependent on the model option selected.
38. This flexibility has the benefit of allowing applicants to use the most appropriate approach for their organisms to demonstrate their products are safe. This would include the latest developments in methodologies as they become available (for example new approach methods (NAMs) or toxicology studies not relying on animal testing or in vitro/clinical allergenicity testing).
39. When the progenitor organism of a PBO for food does not have a history of consumption, assessment in Tier 2 will use the applicable sections of the novel food guidelines, alongside any other data needed to address other triggers that have been met. For all other triggers, suggestions of types of data which may be appropriate for the minimal necessary bespoke assessment may be outlined in other regulatory framework guidelines relevant to the issue that triggered Tier 2. The intention is to require the minimal information necessary to complete the safety assessment and enable decision-making.
2.3 Limitations of the options presented
40. Deciding between Model 1 and 2 is a question of the level of evidence-based assurance required of PBOs by the regulator, building upon those assurances provided by industry due diligence as a starting point. Additional data and evidence potentially correlates to greater assurance that a safety evaluation will identify PBOs with food safety risks, thus allowing these to be reviewed and managed by the FSA.
41. It is noted that allergenicity (particularly from novel proteins) is difficult to assess at the triage stage in both Models. However, this reflects the difficulty of assessing allergenicity more generally, even within existing novel foods regulation. Data required in this space will take account of the latest thinking from the WHO / FAO expert consultation on allergenicity.
42. Choosing to adopt Model 1 would mean there is less information regarding composition, which limits an evidence-based review of all but the most significant phenotypic changes; for example, those that lead to known and expected changes in toxicants, nutrients or allergen content. The presence of unknown and undetected allergens in foods can present a significant food safety risk to some consumers, in all cases (both TBO and PBO). Some specific proteins are the most obvious cause of allergenicity. Some are known, but some may as yet be unidentified. If the introduction of new proteins, or changes to existing proteins, are the intent in the PBO, in Model 1 or 2, this should trigger considerations around the risk management of potential allergy. However, as is the case with novel foods, further quite challenging scientific research is required to perform quantitative risk assessments for allergenicity. If this situation arose for a PBO and there was no history of consumption for the parental organism, one could expect a more thorough assessment of allergenic potential to be triggered.
43. It was highlighted to risk managers that considerations such as time to market or the likelihood of applications being considered under Tier 2 could be influenced by the Model selected. However, this was multifactorial and would also be influenced by the quality of submissions and how the system was operated. These considerations may be helpful to take account of along with other legitimate factors during policy development.
4. Final considerations
In this guide
In this guide50. The ACNFP is presenting two models of data requirement for triage based on differing interpretations of proportionality required in the Genetic Technology (Precision Breeding) Act 2023. There is a choice to be made by risk managers on balancing the level of safety assurance required with the need to deliver on wider policy goals. This will take account of a range of other legitimate factors. The Models are intended to provide an explanation of the current scientific understanding of the technology and how this could inform the decisions of policymakers.
51. It should be noted that most Members of the ACNFP are content with the two Models 1 and 2 proposed in this statement and consider them to offer a realistic choice of data requirements. However, it should be noted a few would prefer still higher levels of technical data for triage (a Model 3 approach) and a few would tolerate no pre-market assessment (a Model 0 approach). All acknowledged that safety assessment can be undertaken, and risk-based decisions can be made even if there are uncertainties and gaps in data and evidence. It is challenging to achieve a consensus on the data requirements in this new area of science and policymaking. New oversight models like those proposed here are required precisely because there is no definitive international consensus on technical guidance for PBO regulation that could be applied here. The context of the policy goals defined in the 2023 Act of Parliament adds another layer of complexity and such goals are likely to vary from nation to nation. This is a rapidly evolving area in the regulation of food safety that warrants ongoing attention.
3. Areas for consideration in implementing the models
In this guide
In this guide44. The ACNFP also considered how the Models could be implemented in order to ensure the data requirements could be practically applied. Some recommendations for consideration by risk managers in deciding on the approach to regulation are outlined below.
45. The Committee considers that the responsibility for the data being provided sits with applicants. Applicants are accountable for the accuracy and conclusion of any statement they provide in support of their application. Being able to navigate an applicant’s argument on how the data presented supports their conclusions on the safety of their product has been important in other regulatory regimes. The Committee recommended that a structured explanatory narrative should present the information and detail supporting the application, the reasoning behind the interpretation of accompanying data and a clear conclusion that answers the requirements. The FSA should reserve the right to request or examine further data and should have powers to seek more data or review where potential risks are identified.
46. Decisions on when and where in a process additional data can be requested from applicants has been key in the effective operation of other regulated product regimes. The Committee recommends that opportunities to request additional data is built into the regulatory process, where pivotal to enable decision-making or where clarification is needed. This should be limited to data expected to be available to the applicant as part of normal due diligence to ensure safety under food law. This holds true for both Model 1 and Model 2.
47. It was commented that where larger data sets are deemed necessary to better understand the safety profile of a PBO, particularly where commissioning of further studies is required, this is more in alignment with Tier 2 of the assessment and would need to be justified as being necessary for decision-making.
48. It was noted that to ensure Tier assignment is working as initially intended, the process may benefit from an audit or review of the first applications after 2-3 years. This could be helpful in establishing precedents and ensuring the guidance is achieving its aims. One approach to achieve this would be that initially all PBO applications are assessed by the ACNFP to ensure the adopted approach is effective and proportionate. Depending on the model chosen by the FSA, this could then move to an approach where the internal FSA science team completes the triage process based on data in initial submissions; applications that require expert advice on more technically challenging aspects would be completed with the support of the ACNFP.
49. Given the potential for the application of technology in this area to evolve quickly, it was suggested that there be a mechanism to ensure the guidance and support materials can be updated. The Committee suggested that the process is subject to regular review every 3 years to ensure the assessment process remains appropriate and fit for purpose in light of technological and political developments.
5. Next Steps
In this guide
In this guide52. Following the anticipated FSA Board decision on which Model and authorisation process to take forward, the ACNFP through the PGT Subcommittee would assist in the development of detailed scientific guidance for applicants on data requirements for assessment of the safety of PBOs for food and/or feed. A co-ordinated approach between the FSA, Defra and ACRE is being developed to provide a consistent experience for applicants. It is anticipated that this process will evolve over time as more experience is gained and it is recommended that the process is reviewed at least every 3 years.
Statement of Interests
In this guide
In this guideThe ACNFP code of practice on declaration of interests and management of conflicts can be found on the ACNFP website; the interests and personal interests are publicly available for each ACNFP Member. This is in agreement with the FSA good practice guidance to ensure interests are declared in a transparent way and managed as required.
Professor Bruce Whitelaw declared financially benefiting from a University of Edinburgh Commercialisation Licence with Genus plc regarding PRRSV-resistant pigs; this was noted and it was agreed that when discussing this particular case study, Professor Whitelaw would be present but only to answer questions on the case.
Glossary
In this guide
In this guideAnticipated Effect – Any effect (desirable or non-desirable) on traits/phenotypes that can be predicted as potentially occurring as a consequence of the intended change. Anticipated effects from the initial submitted data will be considered by the safety assessment process being developed, whereas unanticipated effects (see below) cannot be risk assessed unless evidence emerges.
Cisgenesis – Transfer of genes (which may include their own regulatory elements) from a closely related and sexually compatible donor plant to the genome of a host plant; this could have occurred naturally.
De novo domestication – A recently developed strategy for crop breeding, where domestication-associated allelic variants are introduced into non-domesticated plants. It allows the domestication of elite wild plants while retaining the genetic diversity and associated elite traits and permits design of improved crops in one step where traditional breeding would have required multiple time-consuming crossings.
Donor organism – The source organism of a trait of interest to be transferred to a host organism’s DNA through genetic technology or traditional breeding; in the context of this document, the organism could be a plant or an animal.
Due diligence – Action taken by any actor in the production, processing and distribution of food and feed to ensure all precautions deemed reasonable were taken to avoid a bad outcome and prevent an offence from occurring; due diligence to ensure food safety is mandatory under General Food Law and is, under the Food Safety Act 1990, the best defence for a business to prevent legal repercussions if an incident takes place.
General Food Law (GFL) – The principal aim of retained EU law Regulation (EC) 178/2002, 'General Food Law' is to protect human health and consumer’s interest (Article 5) in relation to food. It applies to all stages of production, processing and distribution of food and feed. General Food law actions are science-based, using risk analysis (Article 6). When risk assessment is inconclusive, the precautionary principle is applied to protect from possible risks (Article 7). Food businesses must comply with food and feed safety law.
Host organism – The final recipient organism of a trait of interest transferred from a donor organism’s DNA through genetic technology or traditional breeding; in the context of this document, the organism could be a plant or an animal.
Novelty – In this context, novelty refers to foods or feeds with no significant prior history of safe consumption in the UK or EU (such foods would fall within novel food regulation (EU) 2015/2283 if they were not precision bred).
Progenitor – Organism from which a plant or an animal is descended or originates.
Traditionally Bred Organism (TBO) – Organism (plants -including algae- and animals) created by the application of genetic principles in agriculture and animal husbandry, carrying developed or improved desirable traits, obtained through a wide range of conservative tools or traditional processes as described in the Genetic Technology (Precision Breeding) Act 2023 (including sexual fertilisation, spontaneous mutation, in vitro fertilisation, polyploidy induction, embryo rescue (plants), grafting (plants), induced mutagenesis (plants), somatic hybridisation or cell fusion of plant cells of organisms which are capable of exchanging genetic material (plants), artificial insemination (animals), embryo transfer (animals), recovery and transfer of primordial germ cells (animals)).
Traditionally bred counterpart – An organism where the same genetic change has been introduced using any conservative tool or traditional processes without the use of precision breeding technologies. This may be a theoretical/conceptual organism and may not be known to exist. The counterpart may be distantly related but will in all cases be sexually compatible.
Unintended effect – A change that was not the objective of the breeding and was not predicted to occur but has occurred and may have consequences for food safety in addition to the intended effect. Unintended effects are inevitable, and also occur in traditional breeding.
List of Annexes
In this guide
In this guideAnnex A. Tables of initial case studies
Annex B. Technical justifications for the data requirements for triaging, assignment to Tier 1 and Tier 2 and for assessment in both Tiers
Annex A. Tables of initial case studies
The Advisory Committee on Novel Foods and Processes (ACNFP) Subcommittee on Products of Genetic Technologies (PGT) used a range of hypothetical example organisms which, based on the academic literature, may be developed and Precision Bred Organism (PBO) status subsequently sought from the Advisory Committee on Releases to the Environment (ACRE). These examples were used to support Subcommittee discussions concerning risks that may arise in PBO-derived products for food and feed use, as well as the development of a framework for the safety assessment of PBOs.
References for the academic literature used to develop hypothetical examples are listed in Tables A and B; these tables will be regularly updated as new examples are used by the Subcommittee.
Table A. Examples of plants, and their traits of interest, which could be produced by precision breeding for potential use in the food/feed industry
|
Host organisms |
Nature of the edited genomic feature, and editing method used |
Potential application |
Reference |
|
Tomato (Solanum lycopersicum L.) |
Genes encoding enzymes of the GABA (Gamma-Amino-Butyric Acid) synthesis pathway
Method: CRISPR/Cas9 |
Tomato with increased GABA (Gamma-Amino-Butyric Acid); potential health benefit (reduced blood pressure, stress relief) |
Nonaka, S., et al. (2017) Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci Rep, 7(1): 7057 https://doi.org/10.1038/s41598-017-06400-y |
|
Cocoa (Theobroma cacao) |
Gene encoding a suppressor of the pathogen defence response
Method: CRISPR/Cas9 |
Cocoa resistant to Phytophthora tropicalis infection; disease resistance |
Fister, A.S., et al. (2018) Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma cacao. Front Plant Sci, 2(9): 268 https://doi.org/10.3389/fpls.2018.00268 |
|
Wheat (Triticum aestivum) |
Genes encoding asparagine synthetase enzymes
Method: CRISPR/Cas9 |
Low asparagine wheat; potential health benefit (reduction of carcinogenic acrylamide production from asparagine during processing) |
Raffan, S., et al. (2021) Wheat with greatly reduced accumulation of free asparagine in the grain, produced by CRISPR/Cas9 editing of asparagine synthetase gene TaASN2. Plant Biotechnol J, 19(8): 1602 to 1613 https://doi.org/10.1111/pbi.13573 |
|
Tomato (Solanum lycopersicum L.) |
Gene encoding a 7-dehydrocholesterol reductase
Method: CRISPR/Cas9 |
Provitamin D3 biofortified tomato fruits for food and tomato leaves for food supplements; potential health benefit |
Li, J., et al. (2022) Biofortified tomatoes provide a new route to vitamin D sufficiency. Nat Plants, 8(6): 611 to 616 https://doi.org/10.1038/s41477-022-01154-6 |
|
The laboratory model plant (Arabidopsis thaliana) but could also be applied to crops |
Gene encoding a chloroplast thylakoid associated protein
Method: CRISPR/Cas9 |
Increased crop oil yield (feed purposes)
|
Bhunia, R.K., et al. (2022) A native promoter–gene fusion created by CRISPR/Cas9-mediated genomic deletion offers a transgene-free method to drive oil accumulation in leaves. FEBS Lett, 596(15): 1865 to 1870 https://doi.org/10.1002/1873-3468.14365 |
|
Cottonseed (Gossypium hirsutum L.) |
Genes encoding products involved in catalysing the desaturation of oleic acid to linoleic acid
Method: CRISPR/Cas9 |
Increased shelf life and oxidative stability of oleic acid in cottonseed oil |
Chen, Y., et al. (2021) High-oleic acid content, nontransgenic allotetraploid cotton (Gossypium hirsutum L.) generated by knockout of GhFAD2 genes with CRISPR/Cas9 system. Plant Biotechnol J, 19(3): 424 to 426 https://doi.org/10.1111/pbi.13507 |
|
Potato (Solanum stoloniferum, Solanum venturii) |
Genes responsible of late blight potato resistance
Method: Random Insertion of cisgenes via marker-free Agrobacterium transformation |
Potatoes resistant to Phytophthora infestans (late blight) disease |
Jo, K.R., et al. (2014) Development of late blight resistant potatoes by cisgene stacking. BMC Biotechnol, 14(1): 50 https://doi.org/10.1186/1472-6750-14-50 |
|
Rice (Oryza alta) |
Genes encoding for grain yield, grain quality, fertility, heading date, biotic and abiotic resistance, and nutrient-use efficiency
Method: CRISPR/Cas9, de novo domestication |
Improvement of six agronomically important traits in a staple cereal; potential benefits for world food production/security |
Yu, H., et al. (2021) A route to de novo domestication of wild allotetraploid rice. Cell, 184(5): 1156 to 1170 https://doi.org/10.1016/j.cell.2021.01.013 |
|
Rice (Oryza sativa japonica) |
Gene encoding the Acetolactate Synthase (ALS), target of Imidazolinone (IMI) herbicides and responsible of interaction with IMI herbicides
Method: CRISPR/Cas9 |
Control of weed proliferation in field by herbicide treatment without concomitant phytotoxicity on rice |
Wang, F., et al. (2021) Creating a novel herbicide-tolerance OsALS allele using CRISPR/Cas9-mediated gene editing. The Crop Journal, 9(2): 305 to 312 https://doi.org/10.1016/j.cj.2020.06.001 |
|
Peanut (Arachis hypogaea L.) |
Genes encoding the Fatty Acid Desaturase 2 (FAD2) enzyme, that converts oleic acid to linoleic acid
Method: CRISPR/Cas9 |
Peanuts with increased oleic acid content for improved oil quality and flavour and improved nut shelf-life; potential health benefit (cardiovascular) |
Neelakandan Anjanasree, K., et al. (2022) CRISPR/Cas9 Based Site-Specific Modification of FAD2 cis-Regulatory Motifs in Peanut (Arachis hypogaea L). Frontiers in Genetics, 27(13): 849961 https://doi.org/10.3389/fgene.2022.849961 |
Table B. Examples of animals, and their traits of interest, which could be produced by precision breeding for potential use in the food industry
|
Host organisms |
Nature of the edited genomic feature and editing method used |
Potential application |
Reference |
|
Chicken (Gallus gallus domesticus) |
Gene encoding the receptor required for avian leukosis virus subgroup J to infect chicken cells
Method: CRISPR/Cas9 |
Chickens resistant to infection by Avian leukosis virus subgroup J |
Koslová, A., et al. (2020) Precise CRISPR/Cas9 editing of the NHE1 gene renders chickens resistant to the J subgroup of avian leukosis virus. Proc Natl Acad Sci USA, 117(4): 2108 to 2112 https://doi.org/10.1073/pnas.1913827117 |
|
Pacific bluefin tuna (Thunnus orientalis) |
Gene encoding a receptor expressed in muscle cells that leads to muscle contraction
Method: TALEN |
Less aggressive tuna not capable of fast swimming in aquaculture; reduction in deaths from collisions with walls |
Higuchi, K., et al. (2019) Targeted mutagenesis of the ryanodine receptor by Platinum TALENs causes slow swimming behaviour in Pacific bluefin tuna (Thunnus orientalis). Sci Rep, 9(1):13871 https://doi.org/10.1038/s41598-019-50418-3 |
|
Pig (Sus domesticus) |
Gene encoding a receptor for Porcine Reproductive and Respiratory Syndrome Virus 1 (PRRSV1)
Method: CRISPR/Cas9 |
Pigs resistant to infection by Porcine Reproductive and Respiratory Syndrome Virus 1 |
C. Burkard, et al. (2017) Precision engineering for PRRSV resistance in pigs: Macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLoS Pathog, 23;13(2): e1006206 https://doi.org/10.1371/journal.ppat.1006206 |
Annex B. Technical justifications for the data requirements for triaging, assignment to Tier 1 and Tier 2 and for assessment in both Tiers
1. In developing the Models for the assessment of the safety of PBOs, the ACNFP-PGT considered case studies (Annex A) and used its expert knowledge to identify scenarios where scrutiny beyond the current due diligence measures for traditionally bred crops and animals would be justified. Based on the discussion, a number of key considerations were identified to inform the development of the information and data required to support tier assignment and safety assessment. The Committee sought to ensure foreseen risks could be identified while ensuring the requirements were proportionate. The key considerations are explored below.
Unintended effects, Intended effects, Anticipated effects
2. When discussing the uncertainties associated with the generation of PBOs, the ACNFP considered whether basic phenotypic information should be requested not only to capture the intended changes but also to provide some evidence to identify any unintended changes in composition. In both Models, information on phenotype as a result of intended changes is requested.
3. Some Members thought that, in the initial stages of PBO authorisation, it would provide additional reassurance and contribute to public confidence if it could be shown that no significant unintended changes in composition are produced. For example, whether new toxins or increased levels of toxins had been inadvertently generated. Conceptually, such unintended consequences are also possible with TBOs, arguably more so if the approach to breeding is not precise and targeted, though not in such short a time frame as with PBOs. However, it was considered by Defra and during the development of the Act, that the history of use of TBOs shows only very rare occurrences of safety concerns as a consequence of unintended changes and these have been managed through due diligence and food and feed law post market.
4. Taking the reasoning of the Act that PBOs could have been generated by TB, by definition, the risk presented by potential unintended changes to PBOs is inherently being considered comparable to TBOs. Some Members of ACNFP remain concerned that the potential for generating traits that are unintended and unknown is there for both TBOs and PBOs, and there is a risk the unknowns are not being covered in either case. However, assessing intended changes, dealing with the knowns and any reasonably anticipated unintended consequence is a pragmatic starting point, appreciating the residual risks that may also be present due to unknowns in a risk management context.
5. There was no suggestion of a need to routinely screen for unintended changes in composition through proteomics, metabolomics, or any other very detailed analysis, given that such monitoring is not done as part of TBO production. It was also highlighted that if such analyses were carried out, it could be difficult to set parameters for identification of an unintended change - particularly a minor change - above the level that could be expected to be found within natural variation. As such changes are no more likely to take place in PBOs when compared to TBOs, the risks associated with unintended changes could reasonably be controlled by due diligence under the GFL. Therefore, Members proposed that only intended changes and unintended changes that could be anticipated be considered, as reflected by the triage questions (Table 1).
6. It would be desirable for applicants to understand and describe any anticipated resulting changes in composition relevant for consumer safety, resulting from, for example:
- genetic changes impacting metabolic or regulatory pathways affecting the nutrient profile associated with such pathways, or potentially impacting known hazards (for example, intentional increases in toxins for pest resistance (for example) could also change the metabolic pathway of other, unrelated toxins and/or allergens);
- loss or change of function of the endogenous DNA at the site of the edit, including downstream effects (for example, this could be the result of the insertion of a cisgene), possibly impacting on additional phenotypes.
7. It was noted that while breeders are aware of the anti-nutrients/toxins that are present in their crops and may monitor this during product development, these might justify greater scrutiny as part of the assessment.
Tissue used in food and/or feed
8. The approach to the assessment being developed is specifically related to edible tissues. For this reason, understanding which parts of the PB plant or animal are destined to be used for food and/or feed can determine whether further scrutiny is necessary (for example, when the genetic change would have phenotypic consequences exclusively in a part of the plant or animal PBO that is not consumed either as part of feed or food, no safety impact would be anticipated on the food and/or feed).
Novelty
9. With regards to novelty, the policy intention is to explicitly remove PBOs from the scope of NF via a consequential amendment to retained Regulation (EC) 2015/2283. Because of this intention, ACNFP was informed that there was a need to ensure there is a clear route for any PBOs which have modified an organism that had not previously been significantly consumed by humans. This would avoid legal loopholes for species where the potential risks are not well understood. These should be subject to the necessary assessment based on that for NF, which has been identified as requiring a deeper level of assessment of risk. As such whether there was a history of consumption of the species modified, was identified as a key parameter for tier assignment.
10. When considering the taxonomic information that could, in part, inform determination of the novelty of a PBO, ACNFP observed that there would be no additional risks associated with a different variety of a commonly eaten species, so differentiation at the species level was preferred. It was noted that this would also align well with the NF regulations, which are also at the species level.
11. While the NF regulation does not include feeds, PBOs for feed that are novel may also present risks and will also need assessment in Tier 2.
12. Changes which are likely to trigger novelty assessment in Tier 2 include those made in the context of de novo domestication of a wild species not commonly consumed: this could raise potential concerns because of uncertainty about composition (including the possible presence of compounds not known to be normally present in the diet) and the nature of any hazards in the host organism. Moreover, de novo domestication would inevitably require multiple genome edits to a wild species in order to obtain the desirable domesticated traits (for example, improvement of crop yield, making the organism or its products more edible/attractive), and the phenotypic differences between the derived PBO and the wild progenitor might further increase uncertainty about composition and potentially impact risk. Additionally, de novo domesticated species could change their adaptation to a certain climate/environment leading to, for example, altered levels of toxic compounds, justifying further scrutiny.
13. It was noted that early identification of applications needing a Tier 2 assessment on the grounds of novelty would enable the FSA to support the applicant to supply relevant data that would assist a subsequent review by the ACNFP.
Nutrition
14. With regards to nutrition, the ACNFP agreed that when a “PBO is designed to introduce significant changes to the nutritional quality of the organism currently consumed that are likely to be disadvantageous to the consumer” (Table 1), it would be important to determine whether further scrutiny of the nutritional quality would be needed in Tier 2 and to provide the evidence base for any risk management that was required.
15. In this context, the greater focus is on deliberate changes to nutrients that may have consequences for the nutritional profile more widely and may result in nutritional disadvantage (this captures both increases and decreases in relevant compounds). Predicted wider impacts on relevant metabolic pathways and the nutritional profile associated with such pathways might justify greater scrutiny (for example, information on the anticipated effects of the manipulation of enzymes involved in the production of secondary metabolites). Known antinutritional factors must also be considered in the assessment, as substantial increases in their level are potential hazards which might pose a greater risk if not identified.
16. Answering the nutrition triage question involves determining the changes in nutritional quality and understanding their impact by comparison to an appropriate reference. For instance, any impact of changes in nutrient profile would also depend on what food is being considered and its contribution to the UK diet as a source of key nutrients (for example, non-staple source would be more likely to be assigned to Tier 1, while staple source might require assessment in Tier 2). Important considerations include:
- How the upper intake levels impact on different sub-populations of consumers.
- Whether increased or decreased levels of nutrients may represent a risk, with potential consequences for all or some consumers.
- Presence of compounds known not to be normally present in the specific food; this should take into account that stacked effects on the diet could result from consumption of several PBOs developed for the same nutritional benefit.
- Whether other foods with similar composition are consumed.
- Wider dietary consideration (for example, information on and an appreciation of the levels of nutrients in an enhanced nutrient crop in the context of other contributions of that nutrient to the diet could avoid the potential for further review; similarly, a decrease in levels of a single amino acid in a PBO might not really be disadvantageous when the rest of the diet is taken into consideration). Information on the protein quality may support consideration of this point.
- It was noted that a mitigating factor for risk managers might be whether the developer intends to market the PBO food via a labelled, identity-preserved route.
17. Any safety concerns regarding intakes to a population subgroup would assign the PBO to a Tier 2 assessment for further review of the impact; however, changes in nutrient profile alone should not be sufficient for allocation to Tier 2; rather, this should depend on the significance of the impact. For example, for an increase (or decrease) to be considered for Tier 2, it would need to be significantly outside the range of current varieties and at the same time represent a potential hazard. To define this significance scientifically and statistically raises the challenge of how best to analyse this and what data/nutritional information would be required to allow comparison. As such significance should be considered on a case-by-case basis.
The question of benchmarking will be further explored when guidelines for applicants are developed.
18. It was suggested that breeders should take steps to be aware of the antinutritional factors that are present in their crops and should monitor this during product development. Any developer marketing a product with increased nutrient levels would likely already have the data to support their claims. Major changes to nutritional quality would also have to be labelled under GFL.
Toxicity
19. Substantial increases in toxic compounds in a crop are a cause for concern above certain levels and would likely require a safety assessment. Therefore, the ACNFP agreed that a PBO “designed to introduce changes that are expected to elevate significantly the toxicity of any foods/feeds derived from the organism” (Table 1) would require further scrutiny in Tier 2; this will capture both intended and anticipated increases in known toxic compounds. To understand potential hazards, information on the following would inform review:
- Whether the target of the change is the organism’s response to pathogens, due to the likely variation in production of toxic compounds frequently known to be produced as part of the organism’s response, which may involve different metabolic pathways.
- Whether the target of the change is stress resistance, due to the potential variation in production of toxic compounds that may be produced under stressful growth conditions.
- Whether the target of the change is an alteration in ion uptake capacity, due to the crossover of use between some essential ion channels in plants and hyperaccumulation of heavy metal contaminants.
20. Important considerations when examining toxicity include:
- Differing impacts can exist for different parts of the population, particularly the most vulnerable groups for example, infants, children, the elderly, those with compromised digestive or immune system based on the consequences that the level of a compound can cause; for PBOs assigned to Tier 2, how toxicity would be managed through marketing could be explored.
- (mitigating factor) High levels of processing can inactivate toxins, reducing concern over their presence. Applicants should reassure themselves that the organism as consumed was subject to effective processing to mitigate this risk.
- (mitigating factor) Presence of toxic compounds in food and/or feed is regulated by GFL, therefore it might be mitigated by testing during development before variety trial and as part of due diligence. However there are no legal limits imposed by UK regulation, except for substances representing a high risk for consumers (for example, erucic acid, mycotoxins; Chemical Food Safety Law).
21. The concern over levels of compounds that might exceed typical levels was explored, raising here again the challenge of how best to define and analyse this. The question of benchmarking will be further explored when the technical guidelines for the applicant are developed according to the chosen Model.
22. It was noted that developers of traditionally bred toxin-containing organisms would typically check levels of toxins throughout product development, but don’t necessarily test for everything in new organisms (for example, potato developers monitoring levels of glycoalkaloids).
Allergenicity
23. How PB could influence the level of allergenicity, particularly of crops, is especially pertinent in the context of the UK population which shows a high prevalence of hypersensitivity and with food allergy being a significant cause of hospital admissions. Substantial increases in the levels of known allergenic proteins would change the allergenic potency of a substance such as pollen or food. This would have the potential to alter the capacity of a substance to initiate new allergies (a process known as sensitisation) or trigger a reaction in an allergic individual (a process known as elicitation). It may also increase the severity of a reaction. Identifying such a potential hazard is crucial to ensure any increased risk is adequately managed. This is relevant to those species that are already known to be allergenic and especially the so-called priority allergenic foods which are listed in Annex II of the Food Information for Consumers Regulation as retained in UK law.
24. Modifying the allergenic potential of an organism is a potential risk posed by any breeding process, and can be further modified by many factors, including abiotic stress, post-harvest management and other food chain production processes. In all cases the risk of elevating the levels of existing allergens would be left to the developer to assess and monitor. Where significant / major changes in levels of allergens can be predicted from the genetic changes made in the PBO, the organism would likely be assigned to Tier 2.
25. The latest expert consultations on risk assessment of food allergens (for example, FAO and WHO. 2022. Risk Assessment of Food Allergens, Part 1; Risk Assessment of Food Allergens, Part 2) provides a risk assessment framework which should be taken into account when considering what would constitute a significant / major change in allergenicity, and in assessing its risk in the context of PBOs.
26. Assessing the risks posed by the introduction of “new” allergenic proteins is currently beset by uncertainty. PBOs, unlike GMOs that involve transgenesis (where a new gene (and hence protein, usually) is introduced into an organism from another organism), would only alter the risk if either the expression of minor allergenic proteins was radically increased or the genetic event in the PBO radically altered the allergenic potential of a protein.
27. In considering whether PB “introduces changes that are expected to alter the allergenicity of any foods/feeds derived from the organism” (Table 1) the applicants should confirm that there are no inadvertent significant changes in allergenic protein levels including, but may not be limited to:
- Whether the host organism for PB is known for its allergenic potential: priority allergenic organisms should receive increased scrutiny at triage, in order to understand any impact on the use of thresholds for allergenic risk management. Knowledge of organisms or products with similar traits in major allergenic food, where tests or history of use have evidenced unchanged allergenicity, may prevent triggering Tier 2 for allergenicity. It was noted that some people do have reactions (including severe reaction such as anaphylaxis) to non-regulated allergenic foods, which might be taken into consideration (Review and validation of Codex priority allergen list, FAO/WHO joint report, 2021).
- Whether modifications to recognised pathways in the PBO may have direct and indirect impacts on allergens (for example, stress and pathogen resistance in particular are traits that are known to increase expression of allergenic proteins and increase the allergenicity of foods and can significantly change allergenicity during post-harvest storage (for example, the presence and potency of an allergen can change in some fruits during storage, maturation and post-harvest)). Such changes may be mitigated if the particular organism is consumed in a processed form which may inactivate allergens and reduce allergenic potency of a food, reducing concern over their presence.
- The design of the PBO needs to consider whether it is likely to result in a radical alteration in protein expression and/or change its allergenic potential; both intended and anticipated increases in known allergenic compounds should be considered.
28. In addition, when the purpose of altering a crop is to reduce its allergenicity (for example, a reduced or allergen-free PBO), the claim should be supported by clinical studies considering the potential to elicit a reaction in sensitive people; a pre-existing, published clinical study of the same trait may support assessment as part of Tier 1.
Other Safety Concerns
29. The question on “any additional features of the PBO that cause food/feed safety concerns” (Table 1) should capture PBOs with changes that may present a greater degree of uncertainty with regards to food and feed safety and that would not be suitably addressed by the nutritional, toxicology, or allergenicity triage questions.
30. Examples of features that may raise other safety concerns include but are not limited to:
- Complex or rare combinations of novel genomic features; with the development of molecular biology techniques, multiple sequential edits are becoming more viable, and may be performed within elite breeding lines rather than donor lines.
- PBOs with stacked PB modifications, each previously authorised individually; these could present new risks.
- Possible pharmaceutical drug interactions (for example, in cases of biofortification).
- Engineering of an organism to produce something that it doesn’t produce normally (rather than a change in level of production).
- Engineering of an organism to produce compounds not known to be normally present in the diet.
31. There are “Other Safety Concerns” where the impact of the trait may alter the degree of novelty that would need to be taken into account to understand any risk, and which may only require limited data to address focused areas of review; these include for example:
- Where an organism is engineered to produce something that it doesn’t produce normally, rather than a change in level of production (for example, by the intentional alteration of a metabolic pathway or pathways; as a result of significant alteration to a protein, changing its properties).
- Where a cisgene from a donor species with no history of safe use has been introduced, and knowledge of the cisgene donor species – particularly in the context of consumed food – would be critical in considering the level of uncertainty). To note, introduction of a cisgene is not considered a scenario of concern by itself, rather the nature of the protein sequence it encodes and whether this might be associated with a hazard (for example, impact on composition) could be important for understanding any potential risk.
- Where an organism is altered to allow a new part of the organism to be consumed, and no plant or animal from the same species provides an history of safe use of the part (tissue), for example where the part of the plant now edible would previously have been toxic.
- Where an organism is altered to allow a change in traditional processing techniques, and there could be concomitant removal of control measures for a hazard (for example, new trait removing the previously required heat processing necessary to allow consumption, and potentially removing control of antinutritional or allergenic compounds and of microbial contaminations).
32. Due to the unforeseen/unanticipated nature of ‘other’ safety concerns that could be identified, the need for a case-by-case approach to additional data that will be required in Tier 2 becomes more acute.
Special considerations for animal feed
33. A single PBO may contribute a more significant portion of an animal diet than it would as part of the human diet, therefore resulting in different level of significance for changes when used as food or feed. Antinutritional factors and digestibility are particularly relevant in this context. The animal(s) the feed is intended for should be identified, as the nutritional needs vary depending on the animal species, for example, ruminants have very different nutritional needs than poultry or pigs.
34. In addition, the part of the plant to be used as feed for animals may be different from that used as food for humans, or the feed might be a by-product of a plant otherwise used to produce non-edible materials. The impact of any change on the edible part of the plant destined for feed should be taken into consideration when assessing a PBO for feed.
Special considerations for animal PBOs
35. Traits introduced precisely to alter the composition of the animal tissues may impact the nutritional content of foods produced from the animal parts. Traits introduced into an animal to allow them to digest a new feed, if they were developed, may also have such an impact.
36. Particular consideration should be given to how PB could affect the composition of products derived from the animal PBO, such as milk from cows or eggs from chicken and other similar staple foods in the diet. Milk and eggs in particular are a key dietary component for young children.
37. Some animals and animal products can contain allergens (for example, fish, shellfish, insects, eggs, milk) and these should also be taken into consideration when determining impact of PB on the quality of foods from animal PBOs.