Statement of the Advisory Committee on Novel Foods and Processes (ACNFP) on Precision Bred Organisms (PBOs) - January 2023
On this page
Skip the menu of subheadings on this page.Introduction
1. The UK Advisory Committee on Novel Foods and Processes (ACNFP) advises the Food Standards Agency (FSA) on matters relating to the safety of products of modern biotechnology destined for food and feed purposes, including products from Genetically Modified Organisms (GMOs) and Precision Bred Organisms (PBOs). The ACNFP provides assurance through evidence and risk-based assessment of food and feed innovation, that food and feed on the market:
- is safe to eat
- does not mislead the consumer
- does not put consumers at a nutritional disadvantage
2. As described in the Genetic Technology (Precision Breeding) Bill, organisms (and the food and feed derived from them) produced by modern biotechnology techniques, such as Genome Editing (GE), that could also have been produced through traditional breeding processes will be classified by Defra as PBOs and will no longer fall under the scope of GMO regulation in England. The scope of the Bill is for precision bred plants and animals. The decision of whether a product of modern biotechnology is a PBO or a GMO lies with the Defra Secretary of State, following the receipt of a report from the UK Advisory Committee on Releases to the Environment (ACRE). Further detail on this process will be released by Defra.
3. Development of the regulatory process will consider how to review the safety of organisms designated as PBOs for food and feed uses in a proportionate manner to ensure consumer safety, whilst taking into account the decision process and supporting evidence requested by ACRE.
4. In addition to the scientific uncertainty that is present in all safety assessments, it is noted that the technology involved in the generation of PBOs is rapidly evolving. This is reflected in the approach being developed by ACNFP for its assessment, which is summarised in this document. The details of this will be provided in the technical guidance for applicants.
5. To support the development of a regulatory approach, the ACNFP provides advice on the current scientific understanding of the safety of food and feed produced by technologies used in precision breeding (PB). In developing the approach to assess PBOs, the ACNFP Products of Genetic Technologies Subcommittee (ACNFP-PGT) discussed the scientific and technical principles that could be used to underpin a proportionate regulatory framework, which were then discussed further with the wider ACNFP membership.
Approach to the ACNFPs consideration
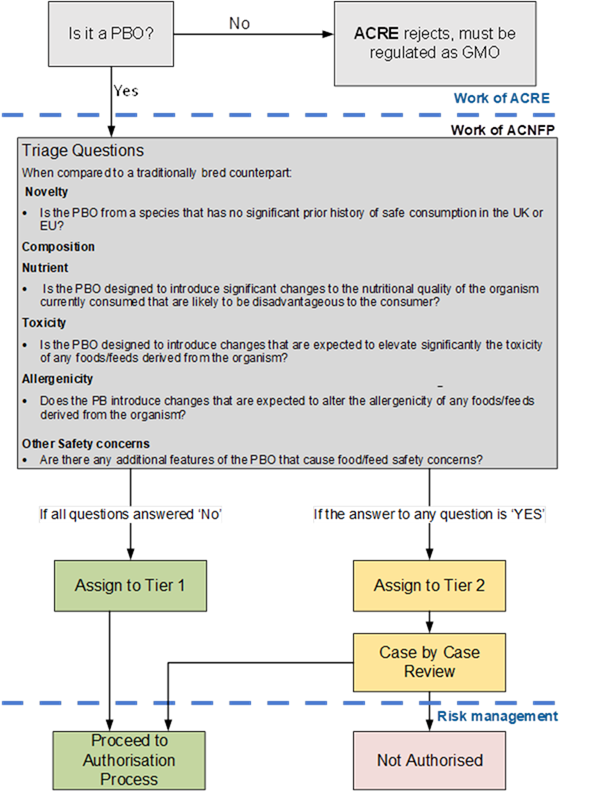
6. The ACNFP released a first statement outlining the context of their discussions. It was noted that when using PB technologies, as with any breeding process, there is the potential to create safety risks for consumers and these need to be identified, assessed, and managed appropriately and proportionately. A two-tiered assessment process for PBOs was therefore suggested, to provide clarity for applicants while allowing appropriate scrutiny of the possible risks as part of the assessment process.
7. To ensure consumer safety, the proposed two-tiered assessment approach has an initial step where PBOs will be triaged based on evidence provided in submissions, and where hazards that ACNFP consider justify a case-by-case review are identified. Further information will be sought at this stage, where needed.
8. In developing the model below, the ACNFP considered available case studies and used their expert knowledge to identify scenarios where scrutiny beyond the current due diligence measures for traditionally bred crops and animals would be justified. There is no evidence that PBOs are intrinsically more hazardous than traditionally bred organisms (TBO). However, it is recognised that a range of outcomes is possible from this rapidly developing technology. These justify scrutiny to ensure that risks are identified and effectively managed.
9. Whilst there is no evidence that the current system of due diligence is ineffective for TBOs, it is noted that the scientific logic underpinning the framework outlined below could also be applied to TBOs meeting similar criteria. In the case of PBOs, the proposed system should reassure producers and consumers that the novelty of the methods involved in PBO production is fully taken account of in the regulatory process. A review to provide scrutiny is justified as there may be opportunities for novel uses of precision breeding technologies, some of which may require a further case-by-case assessment.
The Committee outcome on process and criteria for PBOs
10. To support risk managers in developing a regulatory framework based on expert scientific knowledge, a model for a two-tiered assessment process has been developed and is outlined in Annex A with an overview provided below.
11. Following notification of a PBO from ACRE, a series of triage questions will be used to identify any PBOs that may need further scrutiny due to the potential for increased risk to consumers. This determines tier assignment.
12. The triage process focusses on three themes of particular interest to the FSA: novelty, composition, and other safety concerns.
- Novelty – Organisms determined to be precision-bred for food and feed use will no longer fall within the scope of existing regimes, for example, novel foods or GM. However, it is possible that a PBO will be generated by precision breeding of a progenitor that has not been consumed to a significant degree in the UK or EU whereby further assessment with a similar degree of scrutiny to the novel food approach may be appropriate. This also ensures legislative consistency.
- Composition (which could affect nutrition, toxicity, or allergenicity) – Understanding the genetic changes made during the development of a PBO is essential in determining its safety. Knowledge of the resultant phenotypes and altered traits is important to allow identification of potential for food and feed safety risks to increase or decrease. This allows assessment of intended (anticipated) changes that may be nutritionally disadvantageous for the consumer, as well as any potentially significant changes to the toxicity and allergenicity of food or feed made from the organism.
- Other safety concerns – The use of PB technology may evolve quickly, and, as a consequence, so may the nature of the changes introduced into PBOs for food and feed uses. It is therefore important to establish a mechanism by which PBOs with changes that may present a greater degree of uncertainty with regards to food and feed safety, such as those containing complex or rare combinations of novel genomic features, can be identified and appropriately scrutinised.
13. Those PBOs where the triage process has not identified any food or feed safety hazards above those expected to be present in an equivalent TBO will be assigned to Tier 1, at which point a recommendation can be made for authorisation. Their safety would be assured through the due diligence requirements under the General Food Law for all food and feed to be safe and any other relevant legislation.
14. Should any potential food and feed safety risks be identified in one or more of the triage triggers, the PBO will be assigned to Tier 2, and will be subject to a tailored case-by-case review to allow the identified hazards to be fully assessed.
15.The additional data required, and the nature of the Tier 2 review, will be case-specific and intended to allow understanding of the trigger(s) that prompted a Tier 2 review (i.e., novelty, composition [nutrition, toxicity, or allergenicity], other safety concerns). The ACNFP is developing technical guidance for applicants, so that developers will have clarity on the information likely to be required.
16. Following Tier 2 review, if the concerns have been sufficiently addressed, the scientific assessment would be passed to risk managers to support a recommendation for authorisation. The option for the process not to authorise food or feed for the market remains, for those situations where safety concerns cannot be suitably addressed.
Next Steps
17. The ACNFP through the PGT Subcommittee will pilot the approach using further cases and scenarios to understand the data required to support appropriate safety assessment of a PBO. This information will be used to generate scientific guidance for applicants, in order to provide greater certainty on the expectations of the assessment for each of the triggers. A joined-up approach between Defra and the FSA to deliver their respective responsibilities is being developed to provide a consistent experience for applicants. It is anticipated that this process will evolve over time as more experience is gained.
ACNFP
January 2023
Statement of Interests:
ACNFP code of practice on declaration of interests and management of conflicts can be found on ACNFP website; the interests and personal interests are publicly available for each ACNFP Member. This is in agreement with the FSA good practice guidance to ensure interests are declared in a transparent way and managed as required.
Professor Bruce Whitelaw declared financially benefiting from a University of Edinburgh Commercialisation Licence with Genus plc regarding PRRSV-resistant pigs; this was noted and it was agreed that when discussing this particular case study, Professor Whitelaw would be present but only to answer questions on the case.
Abbreviations
|
Abbreviation |
Definition |
|
ACNFP |
Advisory Committee on Novel Foods and Processes |
|
ACNFP-PGT |
ACNFP Products of Genetic Technologies Subcommittee |
|
ACRE |
Advisory Committee on Releases to the Environment |
|
Defra |
Department for Environment, Food and Rural Affairs |
|
FSA |
Food Standards Agency |
|
GE |
Genome Editing |
|
GM |
Genetic Modification |
|
GMO |
Genetically Modified Organism |
|
PB |
Precision Breeding |
|
PBO |
Precision Bred Organism |
|
PGT |
Products of Genetic Technologies |
|
TBO |
Traditionally Bred Organism |
|
UK |
United Kingdom |
Annex A
Annex A. Proposed decision tree for the safety assessment of food and feed derived from Precision Bred Organisms. Defra, FSA and their scientific advisory bodies are working closely together to ensure their proposed regulatory systems dovetail effectively