2. Committee outcome on the data requirements for Tier 1 and Tier 2 assessment in the context of two models
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.2.1 Proportionality and the options for FSA approaches to data requirements
17. The Genetic Technology (Precision Breeding) Act 2023 confers on the FSA the responsibility to ensure that the regulatory approach to safety for food and feed produced by PB techniques considers “proportionality” as one of the five key underpinning principles of the PB regulation design. The regulatory framework needs to be developed to allow safety assessment of anticipated and specific safety issues associated with PBOs used for food or feed but at the same time support innovation to allow the potential benefits to be realised in a safe and sustainable way. There is therefore a need to consider the minimum data required to ensure that a risk-based safety decision can be made.
18. The ACNFP previously noted that the Act can be interpreted as making an implicit equivalence claim, namely, that TBOs and PBOs have similar risk profiles. This is because PBOs are defined in the Act as organisms produced by modern biotechnology that “could have been produced through traditional breeding processes”. ACNFP Members recognised that most organisms produced by PB will be similar in risk profile to their traditionally bred counterparts, where a safety assessment is not required. However, some organisms produced by TB may also have risks, such as modification of antinutritional factors or alteration of the allergenic potential. These risks are currently managed under due diligence requirements.
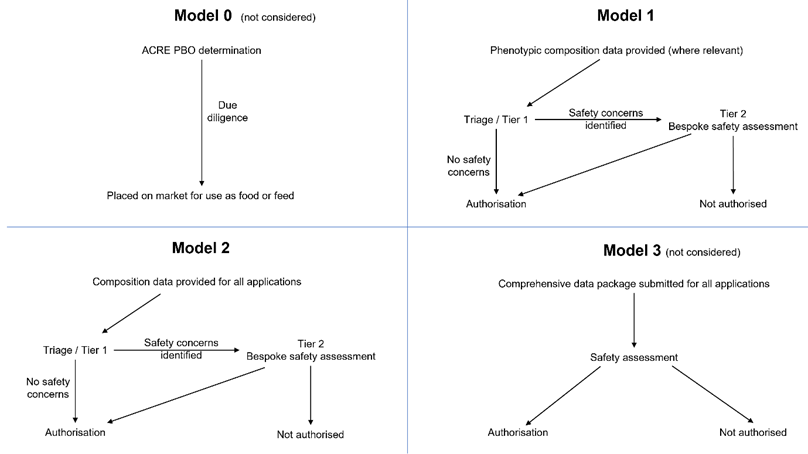
19. Although two scientifically valid models of triage are described in this statements, four models of data requirements could be foreseen (Table 2). ACNFP notes that Model 0 remains a policy option. It would involve no pre-market safety assessment and thus no data requirement for PBOs. However, it was not considered by the ACNFP as its remit was to consider the data requirements for proportionate pre-market safety assessment.
20. A fourth model, Model 3 (Table 2) was briefly discussed by the ACNFP. This would represent an approach to safety evaluation that could provide greater assurance than Model 1 or 2, but it would call for a large battery of compositional and toxicological tests similar to those required for a novel food product. However, this was considered to be excessive for most PBOs. Hence, the focus was given to illustrating data requirements for Models 1 and 2. A schematic representation of the four models can be found in Figure 1.
Table 2. Different models of the approach to triage for the assignment to Tier 1 or Tier 2 during assessment of PBOs
ACNFP has developed both Model 1 and Model 2
|
Triage approaches |
Reasoning of the approach, and extent of the data requirement for triage |
Comment |
|
Model 0 |
PBOs are of equivalent risk to TBOs. (PBOs and TBOs have equivalent safety profile). - No data reviewed, subject to due diligence under the General Food Law. |
For reference only – not considered due to no pre-market safety assessment or data requirement. |
|
Model 1 |
PBOs are equivalent to TBOs, but whether the intended trait is likely to affect food/feed safety needs to be understood. - Information requirement builds on due diligence and is minimal - may include compositional data, but these will not go beyond that required to verify that the intended phenotype has been achieved (for deliberate changes in composition relevant to the quality of food/ feed). The comparator in this model is a TBO with the same or very similar genetic change and phenotypic trait. |
Model identified as scientifically valid – and fulfils policy commission. |
|
Model 2 |
PBOs are equivalent to TBOs, but the innovative nature of the technology used justifies a higher level of scrutiny than Model1, requiring additional compositional data to give a higher level of assurance. - Model 2 builds on the data required to support due diligence and Model 1. In addition, routine data requirements include additional compositional (nutrients/anti-nutrients, toxicology, allergenicity) data. The comparator for the compositional criteria in this model is the organism prior to genetic change. |
Model identified as scientifically valid – and fulfils policy commission. |
|
Model 3 |
Exhaustive assessment of the PBO as a novel food. - Data requirement includes all of the above plus intensive higher tier toxicological or clinical studies (akin to those that might be required for a novel food under that current regulation). |
For reference only – Model identified as not justifiable and may not fulfil the policy commission. |
21. Models 1 and 2 (defined below) diverge on the level of compositional data to be provided in the initial submission to allow for triage into Tier 1 and Tier 2 assessments (defined above). They represent different intermediates in a scale ranging from minimal to more extensive data requirements (Table 2). Data that are considered ‘necessary’ will depend on the level of uncertainty risk managers are content to accept in the safety assessment, and the interpretation of proportionality that policymakers wish to apply when balancing safety assurance and other legitimate factors judged to be within their remit.
- Model 1 focuses on the equivalence between PBOs and TBOs, and on the genetic change and its intended phenotype. The data requirement for safety assessment is predominantly descriptive and confirmatory, with details of the change(s) provided and the description of the resulting product. Compositional data is typically not required in the initial submission. Quantitative data on phenotype is required but mainly focuses on verifying that the intended trait, if relevant to food or feed safety, has been achieved.
- Model 2 builds on Model 1 but focuses on the wider phenotypic consequences of precision breeding and the impact of these on the PBO as consumed. It requires a broader suite of compositional data to be submitted in the initial application. This reflects the view that the new nature of the technology justifies a level of additional scrutiny. Additional to the Model 1 data requirements, compositional data (nutrients and anti-nutrients, metabolite information, proximate analysis (for plants), and edible-by-products data (for animals)) would be routinely required as part of the submission of proposals to inform considerations of any inherent potential for toxicity and / or allergenicity.
Figure 1. Schematic representation of four possible models.
Nature of the data requirements for each, and route between Tiers for the safety assessment of PBOs.
2.2 Data requirements
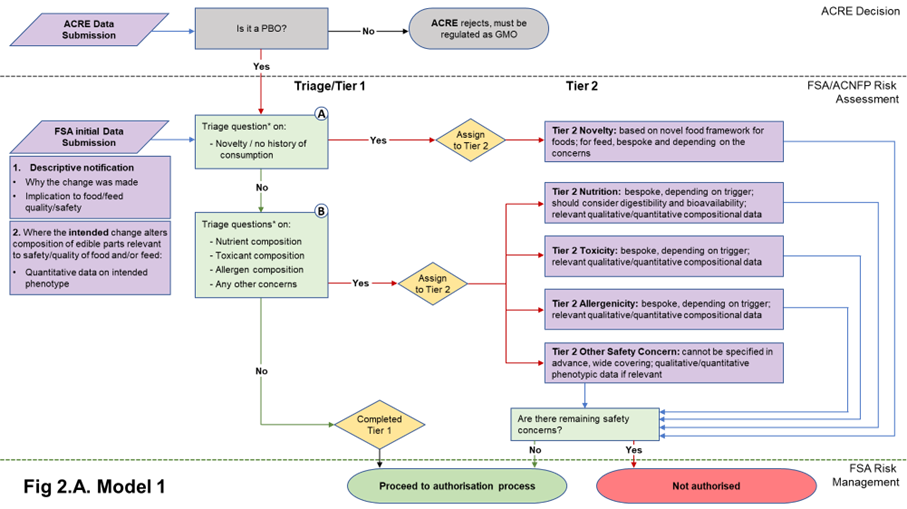
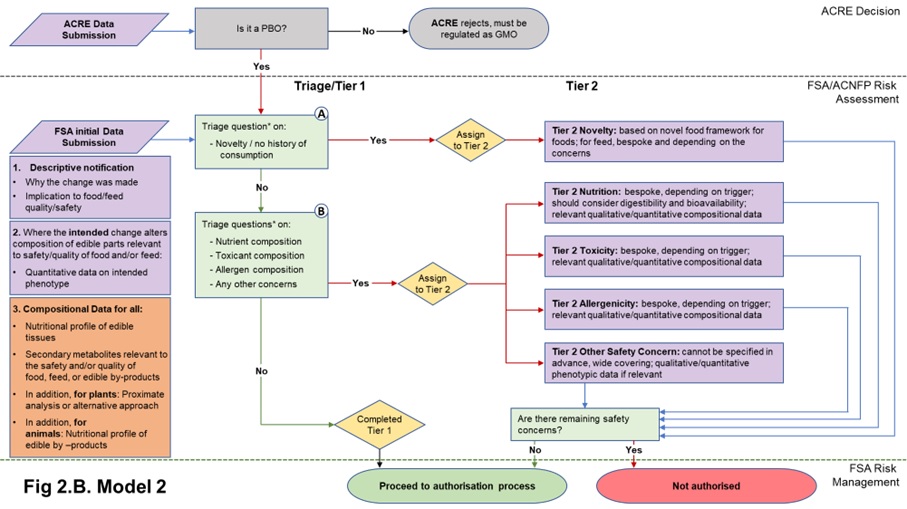
22. The data requirements in both Models 1 and 2 start with a core set of information needed to understand the PBO for which the applicant is seeking authorisation. Model 1 includes a basic composition requirement in the form of quantitative data on the phenotype, to assure the assessor that the PBO is what it says it is. Model 2 also requires this information, plus additional compositional (nutrients, anti-nutrients, metabolites, proximate analysis of edible tissues (plants); nutrients, metabolites of edible tissues and edible by-products (animals)) analysis. The data submitted in the initial submission in either case will be used to answer the triage questions described in Table 1 and determine Tier status. The nature of the compositional data required depends on the model adopted. Flow diagrams representing the two Models and their data requirements are presented in Figures 2A and 2B.
23. In developing the data requirements, the potential risks that might occur were based on the case studies reviewed by the ACNFP (Annex A). This review resulted in a detailed set of technical justifications of the types of data that might be needed in different circumstances. These are explained in detail in Annex B and form the underpinning for the data requirements identified.
Figure 2. Flow diagrams for the regulatory assessment of PBOs.
Figure 2. (A) In Model 1, the focus is the nature of the genetic change and its immediate phenotypic consequences. (B) In Model 2, the focus is on the wider consequences of the genetic change. PBO status is determined by ACRE; FSA / ACNFP conduct safety assessment to advise on safety for authorisation for use as food/feed. In addition to descriptive information (1) and compositional data on the trait (2) required in Model 1, Model 2 also requires additional compositional and nutritional data (3). When safety concerns are identified during steps A and B of the assessment (triage questions* listed in Table 1), applications are assigned to Tier 2, where data requirements are on a case-by-case basis. Purple/orange: data submission; green: data assessment; yellow: outcome of triage.
2.2.1 Non-compositional descriptions of all PBOs in the initial data submission
24. A non-compositional initial submission is required of all applications and would be the same for both Model 1 and Model 2. This will consist of a description of the identity of the PBO and its characteristics (i.e., how it compares to its traditionally bred counterpart, and the hazards or risks it may present) to allow triage. This information builds on what is expected to be required for submission to ACRE for determination of PBO status but should be tailored for suitability to food and feed safety assessment.
25. In order to understand the genomic change made to create the PBO, the applicant should provide information on:
- The target gene(s), i.e., name(s) and primary function(s) together with the reason for targeting.
- Description of the impact of the genetic change and how it achieves its mode of action.
- The intention or purpose for making the change.
- The materials and methods used to obtain the alteration, including evidence that any edit was made where intended, and a description of the analysis or procedures undertaken to minimise the potential for unintended alteration of the organism’s genetic material (so-called “off-targets”), and confirmation of the absence of relevant vector-derived sequences.
- Description of the predictable effects of the genetic change on genomic features at the site of insertion of a cisgene.
26. To further support the understanding of the PBO as a food or feed, the following information should be provided:
- Identification of the parts destined for food and/or feed use, and;
- Intended use, for example, food and/or feed; the animal(s) for which a feed would be intended should be identified. Information on the contribution of the food to the overall diet of the population and information on likely processing before use for food and/or feed may be helpful.
27. To allow determination of the novelty of the PBO, based on the associated triage question (Table 1), applicants must identify whether the organism subject to the -precision breeding or products derived from it have a significant prior history of safe consumption in the UK or EU. The applicant should provide:
- Taxonomic information on the organism (Family, Genus, Species) and a statement on the history of safe use of the PBO species relating to food and/or feed use.
28. In addition to the information identified in the data requirements, some information was flagged as providing helpful context, if available to applicants. Although all organisms without a history of consumption (novelty, as defined above) would require Tier 2 assessment, not all new traits introduced to a species would require further scrutiny in Tier 2. A trait new to a PBO may have a history of safe use from a closely related species with a similar role in the diet. Information which could be provided to support this may include, but not limited to:
- Information on homologous genes in closely related organisms (where the function of an introduced cisgene is novel to the host species), and;
- Information on food and/or feed products or organisms already on the market containing an equivalent trait or mutation in homolog gene(s), and with the same function in the diet.
29. It is recommended that applicants be asked to provide comprehensive information in a statement reviewing how the introduced trait could impact the quality of food and/or feed. They should also offer their scientific evaluation and conclusion in considering how nutritional quality and safety profile may be significantly altered.
30. The technical justifications presented in Annex B highlight particular areas of concern for the safety of PBOs for food and/or feed, which may support the applicants in identifying information that would be relevant to submit. The type of information provided could include, but not limited to:
- Where loss, gain, or change of function of the endogenous DNA and/or other “on-target” impacts in addition to the precise intended edit were identified at the site of the genetic change, evaluation of the likely impact relevant to the quality of food and/or feed, based on the available knowledge. For animal PBOs, when limited information is available on the function of the endogenous DNA, information on the function of homologs in other organisms.
- Description of the intended trait.
- Description of predicted changes in physiology of the plant or animal.
- Description of metabolic or regulatory pathway(s) with which the genetic change may be anticipated to interfere, including the resulting potential impact on the quality of food and/or feed, based on the available genetic and physiological knowledge; for animal PBOs, where functional genetics is less advanced, knowledge of the function of homologs in other organisms may be relevant.
- Information on any likely significant alteration in protein expression and/or change in its allergenic potential.
- Confirmation that levels of antinutrients, toxins or allergens known to the host species are not expected to be affected by the change.
2.2.2 Compositional data relevant to the quality of food and/or feed in initial data submission in Model 1
31. By principle, the Model 1 approach to triage and Tier 1 assessment focusses on the genetic change and its intended phenotype. Some phenotypic changes introduced into a PBO may not be relevant to the quality of food and/or feed. Understanding such relevance will allow focussed scrutiny where necessary and avoid analysis of information irrelevant for food safety. It was noted that information or data to support this should already be available to the applicants and should have been used to underpin their reasoning when ensuring due diligence to comply with GFL.
32. Where the intention of the PB is to intentionally change the composition in the PBO in a way that is likely to affect the quality of food and/or feed, the applicant should provide:
- Compositional data to demonstrate that the desired phenotypic change has been achieved. This is both to understand the significance of a phenotypic change relevant to the quality of food and/or feed, and to ensure that a PBO for food and/or feed “is what it says it is”. For Model 1, no further compositional data should be required as part of Tier 1.
2.2.3 Additional compositional data in Model 2 of triage in the initial data submission
33. The Model 2 approach to triage and Tier 1 assessment focusses on wider phenotypic consequences of the genetic change, as opposed to solely those which were intended. This requires understanding any compositional changes relevant to food and/or feed in the organism. Whilst requiring more data than Model 1, the aim remains to keep the additional data requirement to a minimum while providing a higher degree of assurance by comparison to Model 1. Other models are available that would provide further assurance but these were not considered in detail, as explained above.
34. Model 2 data requirements build on those of Model 1, i.e., data on deliberate compositional changes introduced by PB. In addition, further compositional data relevant to the context of the genetic change (i.e., depending on the host organism and what can be anticipated from the nature of the induced change) is required. This additional requirement differs for plants and animals, due to the wider range of phenotypes possible in plants, compared with animals.
- For plants: for the edible part(s), applicants should provide nutritional profile, proximate analysis (including vitamins, minerals for example) or alternative approach, levels of secondary metabolites relevant to the species or the anticipated changes, which are pertinent to nutrition, toxicology or allergenicity. This will provide a high-level compositional profile or specification for the organism.
- For animals: the focus would be on the composition of all derived edible products, not only the tissues of the PBO but also for example, milk or eggs. Nutrient profile, levels of secondary metabolites relevant to the species or to the anticipated changes which are pertinent to nutrition, toxicology or allergenicity would need to be provided for these derived food products.
35. Data submission should be tailored to the composition profile of the PBO and consider known hazards, to enable the questions relating to triage to be answered effectively, using appropriate reference databases.
2.2.4 Data requirements for Tier 2 assessment
36. Where the information provided in the initial data submission elicits a positive (‘yes’) answer to any of the triage questions (Table 1), or where there is insufficient information to understand the possible safety concerns presented by a PBO, further assessment will be triggered as part of a Tier 2 assessment.
37. Each triage question triggers a specific Tier 2 assessment: the data requirements for Tier 2 assessment will depend upon the factors which identified a need for further scrutiny, i.e., bespoke and considered on a case-by-case basis. Therefore, there cannot be a predetermined list of data requirements or tests for Tier 2. However, technical guidance will be developed for the applicants in terms of what should be provided in the initial assessment dependent on the model option selected.
38. This flexibility has the benefit of allowing applicants to use the most appropriate approach for their organisms to demonstrate their products are safe. This would include the latest developments in methodologies as they become available (for example new approach methods (NAMs) or toxicology studies not relying on animal testing or in vitro/clinical allergenicity testing).
39. When the progenitor organism of a PBO for food does not have a history of consumption, assessment in Tier 2 will use the applicable sections of the novel food guidelines, alongside any other data needed to address other triggers that have been met. For all other triggers, suggestions of types of data which may be appropriate for the minimal necessary bespoke assessment may be outlined in other regulatory framework guidelines relevant to the issue that triggered Tier 2. The intention is to require the minimal information necessary to complete the safety assessment and enable decision-making.
2.3 Limitations of the options presented
40. Deciding between Model 1 and 2 is a question of the level of evidence-based assurance required of PBOs by the regulator, building upon those assurances provided by industry due diligence as a starting point. Additional data and evidence potentially correlates to greater assurance that a safety evaluation will identify PBOs with food safety risks, thus allowing these to be reviewed and managed by the FSA.
41. It is noted that allergenicity (particularly from novel proteins) is difficult to assess at the triage stage in both Models. However, this reflects the difficulty of assessing allergenicity more generally, even within existing novel foods regulation. Data required in this space will take account of the latest thinking from the WHO / FAO expert consultation on allergenicity.
42. Choosing to adopt Model 1 would mean there is less information regarding composition, which limits an evidence-based review of all but the most significant phenotypic changes; for example, those that lead to known and expected changes in toxicants, nutrients or allergen content. The presence of unknown and undetected allergens in foods can present a significant food safety risk to some consumers, in all cases (both TBO and PBO). Some specific proteins are the most obvious cause of allergenicity. Some are known, but some may as yet be unidentified. If the introduction of new proteins, or changes to existing proteins, are the intent in the PBO, in Model 1 or 2, this should trigger considerations around the risk management of potential allergy. However, as is the case with novel foods, further quite challenging scientific research is required to perform quantitative risk assessments for allergenicity. If this situation arose for a PBO and there was no history of consumption for the parental organism, one could expect a more thorough assessment of allergenic potential to be triggered.
43. It was highlighted to risk managers that considerations such as time to market or the likelihood of applications being considered under Tier 2 could be influenced by the Model selected. However, this was multifactorial and would also be influenced by the quality of submissions and how the system was operated. These considerations may be helpful to take account of along with other legitimate factors during policy development.