Cellobiose Discussion Paper
On this page
Skip the menu of subheadings on this page.Committee Paper for Discussion - ACNFP/157/10
Advisory Committee for Novel Foods and Processes
Application for authorisation as a Novel Food for Cellobiose.
Application Number - RP1109
Issue
An application has been received under the novel food authorization process (regulation 2015/2283 as repatriated) for cellobiose, a disaccharide to be used as an ingredient to replace sugars, with the function of a low calorie sweetener.
The Committee is asked to advise on whether the available data provides an adequate basis for a risk assessment, and whether the novel food is safe and not nutritionally disadvantageous under the proposed use and use levels.
Background
1. On May 12th 2021, the FSA received the submission for Cellobiose, a sugar replacement ingredient. The novel food ingredient is a disaccharide consisting of two glucose units linked by a β-1-4 glycosidic bond obtained from two-step enzymic conversion of sucrose into cellobiose. The applicant intends to use the novel food as an ingredient in various food categories to partially replace sugars such as sucrose or lactose.
2. The application dossier is attached as Annex A which is a revised copy from the applicant, incorporating responses to requests for further information made during the suitability check. The request (with systematic responses) are also attached in Annex B. Supporting documents for all sections of the application can be found in Annex C. All annexes contain confidential information.
This application
Identification
3. The novel food ingredient consists of two glucose monomers linked through a β1,4-glycosidic bond. The identity was initially verified by 1H NMR and later through NMR analyses confirming the beta-glycosidic bond between the two glucose moieties that is characteristic for cellobiose. Molecular mass of the NF was analysed by mass spectrometry which showed high congruence with the reference molecular mass of 342.162, further confirming the identity of cellobiose. All certificates can be found in Annex C: 2.1.
4. Physico-chemical analyses (Table 1 below) shows that cellobiose acts similarly to sucrose in Maillard reactions and is thus seen as a valuable ingredient in heated meat products.
Table 1: Physicochemical properties of cellobiose
|
Property |
Value |
Reference |
|
Appearance |
White powder |
GBA analysis |
|
Melting point |
236.24 °C |
GBA analysis |
|
Boiling point |
- |
|
|
Relative density |
1.04 kg/L |
GBA analysis |
|
Solubility |
111 (g/L at 15°C 118.9 g (g/L at 30.5°C) |
(Yalkowsky et al., 2010) |
|
Refraction index |
1.35 °Brix |
GBA analysis |
|
Optical rotation |
34.66 ° |
GBA analysis |
|
pH |
4.26 |
GBA analysis |
|
Water activity |
0.47 |
GBA analysis |
Production Process
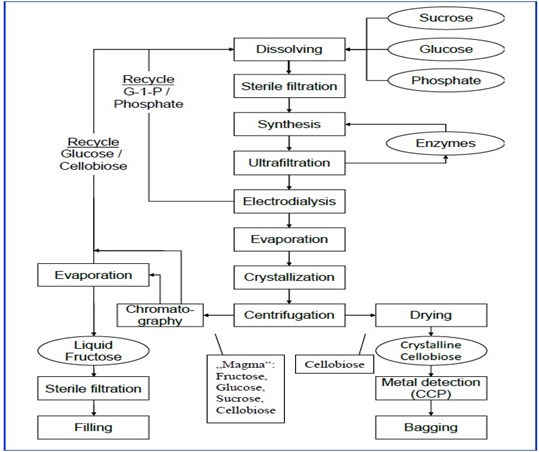
5. The applicant states that the production process involves two enzymes, sucrose phosphorylase and cellobiose phosphorylase where the first converts sucrose into fructose, the by-product of the process, and G1P. This intermediate is the substrate of the second enzyme that synthesizes cellobiose by coupling one molecule glucose to one molecule of G1P via a ß-1-4 glycosidic bond.
6. Cellobiose is separated from the enzymes by ultrafiltration, with further purification via electrodialysis. The final product is obtained by crystallization. The applicant states there are no unwanted side products formed. Figure 1 below shows the manufacturing flow scheme for cellobiose with the full process (confidential) detailed in Annex A: dossier p13-15 and Annex C: 2.3.
Figure 1: Manufacturing flow scheme for cellobiose.
7. The applicant also clarifies on cellobiose originally being developed as a feed ingredient, that following their decision to launch it as a food, 5 batches were manufactured and reserved for the analysis and studies that are mandatory for novel foods. The applicant has confirmed that the cellobiose the subject of this application is exclusively manufactured to food grade quality.
Composition
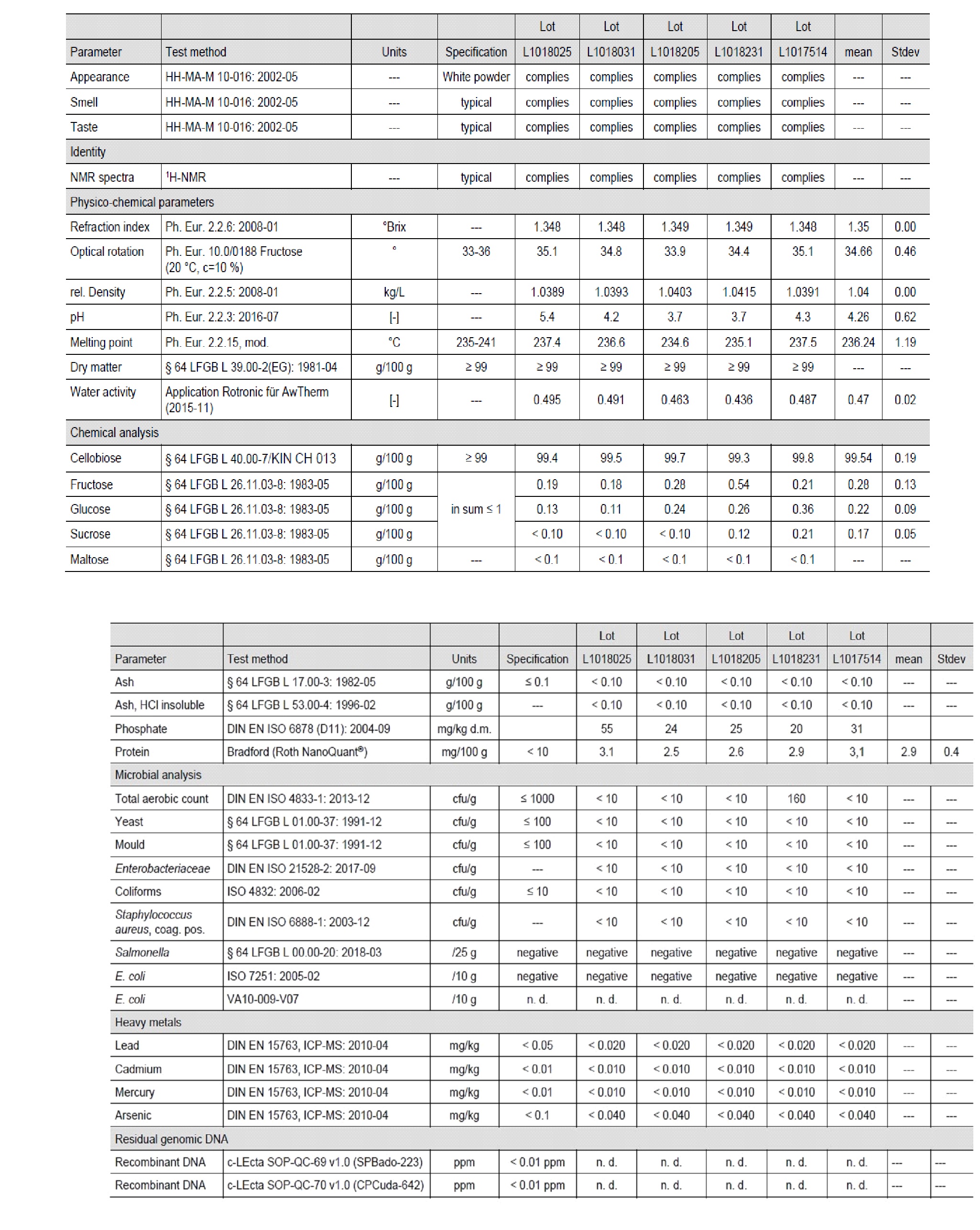
8. The applicant has reported analytical data for 5 independent batches of the novel food ingredient. They also state that analysis of cellobiose is done by third party laboratories with a list given. All accompanying CoAs, accreditations and methods can be found in Annex C: 2.4.
9. The analysis parameters includes chemical, physio-chemical, microbial, heavy metals and residual genomic DNA as seen in Figure 2 below and Annex A: p1920.
Figure 2: Analyses of five independent batches of cellobiose (n. d. = not detected --- = not applicable)
Stability
10. The applicant states that stability studies were conducted at normal storage conditions (25 °C/60% RH) and accelerated conditions (40 °C/75% RH) by a third-party laboratory with the key parameters being microbial, chemical and sensory all over 24-month period (Table 2 and Annex A: p21). CoAs for the tests can be found in Annex C: 2.4.07-2.4.11.
Table 2: Sampling scheme for stability assays cellobiose.
|
|
Storage condition |
|
Sampling and analysis (months) |
|
|
|
|
||||
|
Cellobiose |
25 oC/60% RH |
Initial analysis |
- |
1 |
- |
3 |
6 |
9 |
12 |
18 |
24 |
|
|
40 °C/75% RH |
|
0.5 |
1 |
2 |
3 |
6 |
- |
- |
- | - |
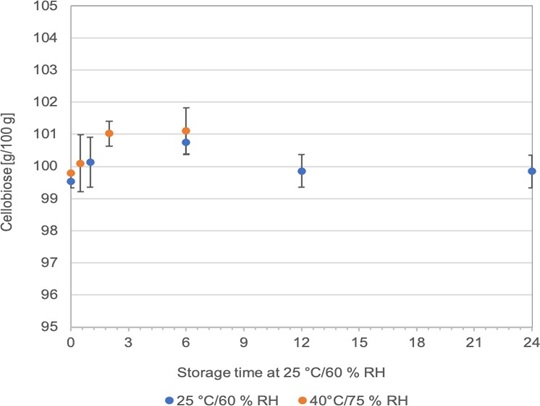
11. The applicant reports that cellobiose was stable for at least 24 months at normal conditions (25 °C/60 % RH) and at least 6 months at accelerated conditions (40 °C/75 % RH) as shown in Figure 3 below, Annex A: p22. They state that glucose concentrations remained below 1% under both test conditions. All results can be found in Annex C: 2.4.12.
Figure 3: Cellobiose stability at 25 °C/60 % RH over 12 months and at 40 °C/75% RH over 6 months; average values and standard deviation are given, n=5.
12. They report that water activity remained constant under normal conditions and increased from 0.47 to 0.53 at accelerated conditions (Table 3 and 4 below, Annex A: p22-23) and that this was still low enough to inhibit microbial growth throughout.
Table 3: Water activity data for stability tests with cellobiose at 25°C ± 2°C/60% RH ± 5% RH
|
|
L1017514 |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
Average |
SD |
|
Start |
0.49 |
0.50 |
0.49 |
0.46 |
0.44 |
0.47 |
0.02 |
|
1 month |
0.37 |
0.39 |
0.38 |
0.45 |
0.44 |
0.41 |
0.03 |
|
3 months |
0.52 |
0.51 |
0.48 |
0.52 |
0.56 |
0.52 |
0.03 |
|
6 months |
0.48 |
0.45 |
0.46 |
0.50 |
0.54 |
0.49 |
0.03 |
|
9 months |
0.39 |
0.44 |
0.35 |
0.43 |
0.52 |
0.43 |
0.06 |
|
12 months |
0.48 |
0.53 |
0.46 |
0.54 |
0.54 |
0.51 |
0.03 |
|
18 months |
0.36 |
0.37 |
0.34 |
0.47 |
0.38 |
0.38 |
0.04 |
|
24 months |
0.48 |
0.46 |
0.42 |
0.49 |
0.49 |
0.47 |
0.03 |
Table 4: Water activity data for stability tests with cellobiose at 40°C ± 2°C/75% RH ± 5% RH
|
|
L1017514 |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
Average |
SD |
|
start |
0.49 |
0. 50 |
0.49 |
0.46 |
0.44 |
0.47 |
0.02 |
|
0.5 months |
0.38 |
0.62 |
0.59 |
0.57 |
0.66 |
0.56 |
0.10 |
|
1 month |
0.60 |
0.37 |
0.48 |
0.4 |
0.47 |
0.46 |
0.08 |
|
2 months |
0.65 |
0.45 |
0.46 |
0.50 |
0.54 |
0.52 |
0.07 |
|
3 months |
0.53 |
0.58 |
0.47 |
0.60 |
0.63 |
0.56 |
0.06 |
|
6 months |
0.55 |
0.50 |
0.45 |
0.56 |
0.59 |
0.53 |
0.05 |
13. They also report that microbial quality assessed did not show an indication of microbial growth under both storage conditions ( Annex C: 2.4.12) and neither did the colour have major changes (Annex C: 2.4.07-2.4.11).
Specification
14. The applicant states that following the processing method, no other sugars besides cellobiose are expected in the product. Cellobiose is analysed by HPLC and identified by its retention time, which is then compared with a reference standard. They also deem cellobiose and glucose to be suitable parameters to characterize and substantiate the purity of their product. With the absence of specific requirements for sugars within the category the NF is intended for, they set the specifications according to its manufacturing capabilities. The specification CoA can be found in Annex C: 2.5: 2.5.1.
History of Use
15. The applicant states that sucrose and glucose both have a long history of safe use as a food ingredient and are present in foods such as vegetables, fruits and honey. However, they note that pure cellobiose has not been used as a food ingredient before.
16. Through the literature review, the applicant found that honey has trace amounts of cellobiose at levels of 0.06–0.28 g/100g and developing maize grains at levels of up to 0.05 mg/g in the embryo and 0.06-0.13 mg/g in the endosperm. They also state that nutritionally relevant concentrations of the NF have not been detected in food.
Proposed Use and Intake
17. The applicant states that the novel food ingredient is intended to be used by the general population excluding infants under 1 year of age.
18. The novel food is intended to be used as an ingredient in several categories to replace sucrose or lactose, or function as a low calories sweetener (Table 5 below, Annex A: p30-31). They further explain that due to the NF being a substitute, the dietary intake of cellobiose resulting from the intake of the listed foods is a matter of dietary preference and as such only maximum proposed use levels are provided.
Table 5: Food categories and intended use levels for cellobiose [mg/100 g]
|
FAIM |
FoodEx |
Food category |
Proposed maximum use levels [g/100 g] |
|
5.4 |
Decorations, coatings and fillings, except fruit-based fillings covered by category 4.2.4 (fondant and sugar-based biscuit coatings) |
|
60.00 |
|
|
A035N |
Basic sweet masses |
60.00 |
|
8.2 |
Meat preparations as defined by Regulation (EC) No 853/2004 (only in case added sugar is applicable) |
2.00 |
|
|
|
A0EYQ |
Marinated meat |
2.00 |
|
8.3 |
Meat products (only in case added sugar is applicable) |
2.00 |
|
|
8.3.1 |
Non-heat-treated processed meat (only in case added sugar is applicable) |
2.00 |
|
|
|
A022R |
Raw cured (or seasoned) meat |
2.00 |
|
|
A024G |
Fresh raw sausages |
2.00 |
|
|
A022L |
Animal meat dried |
2.00 |
|
8.3.2 |
Heat-treated processed meat (only in case added sugar is applicable) |
2.00 |
|
|
|
A023G |
Cooked cured (or seasoned) meat |
2.00 |
|
|
A0EYP |
Preserved or partly preserved sausages |
2.00 |
|
A026K |
Meat based spreadable-textured specialities |
2.00 |
|
|
A026M |
Liver based spreadable-textured specialities |
2.00 |
|
|
A024B |
Canned-tinned meat |
2.00 |
|
|
11.4.3 |
Table-Top Sweeteners in tablets |
60.00 |
|
|
|
A0F7X |
Table-top sweeteners in tablets |
60.00 |
|
12.2.2 |
Seasonings and condiments |
40.00 |
|
|
|
A16GK |
Savoury sauce dry preparation |
40.00 |
|
14.1.4.1 |
Flavoured drinks with sugar |
1.50 |
|
|
|
A03EA |
Soft drink, with fruit juice (fruit content below the minimum for nectars) |
1.50 |
|
|
A03EX |
Soft drink, flavoured, no fruit |
1.50 |
|
A03FQ |
Cola-type drinks |
1.50 |
|
|
14.1.4.2 |
Flavoured drinks with sweetener |
2.50 |
|
|
A03EA |
Soft drink, with fruit juice (fruit content below the minimum for nectars |
2.50 |
|
|
A03EX |
Soft drink flavoured, no fruit |
2.50 |
|
|
A03FQ |
Cola-type drinks |
2.50 |
|
|
17 |
Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children |
80.00 |
|
|
A03SJ |
Food supplements and similar preparations |
80.00 |
|
|
Additives authorised for use in Food flavourings |
100.00 |
||
|
Food additives in nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E of Annex II |
9.5 |
19. The applicant proposes a maximum daily intake of 0.44 g/kg bw/day, with single doses of not more than 0.26 g/kg bw per day based on the human tolerance study of cellobiose (Annex A: 2.10.3.3). The resulting proposed maximum daily intake is summarized in Table 6 below. These range from 15g/kg in flavored milk to 600g/kg in table sweeteners. The applicant notes that the proposed maximum intake for infants, toddlers and young children is derived from a study for adults and as such should be interpreted with caution.
Table 6: Proposed maximum intake of cellobiose, based on the human tolerance Study conducted by the applicant
|
Population Group |
Age |
Bw [kg] |
Proposed maximum daily intake [g] |
|
|
single dose 0.26 g/kg bw/day |
overall daily intake 0.44 g/kg bw/day |
|||
|
Infants |
< 12 m |
6.0 |
not applicable |
not applicable |
|
Toddlers |
12-35 m |
11.9 |
3 |
5 |
|
Other children |
36 m - 9 y |
23.1 |
6 |
10 |
|
Adolescents |
10 y – 17 y |
50.0 |
13 |
22 |
|
Adults |
18 y – 64 y |
73.9 |
19 |
32 |
|
Elderly and very elderly |
> 65 y |
75.0 |
19.5 |
33 |
20. They also note that even though cellobiose is not a dietary fibre, it may act as a prebiotic and that the adequate intake of dietary fibre for young children (2 g/MJ) was derived from the adequate intake for adults (2-3 g/MJ, corresponding to 25 g) according to EFSA Panel on Dietetic Products Nutrition and Allergies (2010). Subsequently, the assumption that cellobiose ingestion will not cause a nutritional dysbalance in the younger population is made.
21. The applicant does not consider other natural sources to significantly contribute to cellobiose intake hence the mean and high daily intake proposed are representative for total intake of the NF from all sources. They conclude that there are no concerns regarding contamination or exposure to undesirable substances. Additionally, cellobiose should not be used for those under 1 year due to lack of safety data.
Absorption, Distribution, Metabolism and Excretion (ADME)
22. Following a systematic literature review on cellobiose in humans, the applicant concludes that the NF is not absorbed in the human intestine in significant quantities although a slight increase in coeliac disease is noted. They also note there is no effect on blood sugar or insulin secretion and that digestibility of cellobiose might be different in populations with different lactase activities due to geographic origin. Additionally, that there is no data post 1987, with available data allowing for an estimated available energy content of 2kcal/g for cellobiose.
23. The applicant also sponsored a series of experiments to investigate the prebiotic properties of cellobiose with tests on stability of cellobiose in gastric pH conditions revealing that there was no release of reducing sugars or glucose containing cellobiose and inulin as substrate whereas starch was hydrolyzed. Another experiment to study the resistance of cellobiose to brush border cellderived enzymes and to intestinal absorption showed that the NF was not significantly degraded by the enzyme of the cell. Full description and results of the experiments performed can be found in Annex C: 2.8: 2.8.1.
Nutritional Information
24. The applicant investigated on nutritional information via literature review and reported that the NF is not degraded nor absorbed in the human intestine, has an influence on microbiota, fermentation releasing acids and formation of short chain fatty acids. Additionally, cellobiose was given an energy value of 2kcal/g in comparison to similar fructo-oligosaccharides and lactulose.
25. The applicant concludes the NF in not nutritionally disadvantageous and is intended to partially substitute sugar in foods with high sugar, substitute lactose in different applications or as a carrier for other substances. They also state that cellobiose will be properly labelled on the ingredient list. The nutritional profile is provided in Table 7 below.
Table 7: Nutritional profile cellobiose
|
Nutritional information |
Unit |
per 100 g cellobiose |
|
Energy |
kcal |
200 |
|
Total fat |
g |
0 |
|
Carbohydrates |
g |
100 |
|
Sugars |
g |
1 |
|
Glucose |
g |
0 |
|
Cellobiose |
g |
99 |
|
Protein |
g |
0 |
|
Salt |
g |
0 |
Toxicological Information
26. The applicant carried out a systematic literature review on the toxicity of cellobiose considering the effect on intestinal permeability, its importance for biofilm formation and virulence of K.pneumoniae.
27. Proprietary toxicological studies were performed on the NF which included a mutagenicity OECD471 report resulting in no effects (full report in Annex C: 2.10.2.1.1), a genotoxicity OECD487 report using 2000µg/ml max concentration resulting in no effects (full report in Annex C: 2.10.2.1.2).
28. A sub-chronic oral toxicity done in a 28-day dose range finding administered to 12 male and 12 female rats in 4 groups using +0%, 5%, 10%, 15% of the NF resulted with the male having reduced body weight for 10-15% NF and female for 15% (full report in Annex C: 2.10.2.3.1). A 90-day study OECD408 report that involved 50 male and 50 female rats in 4 groups using +0%, 2.5%, 5%, 10% of the NF resulted with the male having reduced body weight at 5% and 10% and a decrease of food consumption from 5% (full report in Annex C: 2.10.2.3.2).
29. The applicant performed supplementary analysis of OECD408 to inform on reduced food consumption and body weights as per EFSAs request, concluding that there were no adverse effects related to exposure (NOAEL) ≥10% cellobiose in drinking water equivalent to 6852 and 8043 mg cellobiose / kg bw for males and females respectively (full report in Annex C: 2.10.2.3.2). Based on the results of all the studies, the applicant also concluded that no other studies were needed.
30. Human studies on cellobiose via literature review resulted in no effects on blood sugar and insulin secretion, increased breath hydrogen and no occurrence of diarrhoea on intake of cellobiose was calculated to be 0.36g/kg body weight with the ED50 observed to be 0.62g/kg bw similar to lactulose (Annex A: p58-60). Proprietary studies performed in healthy subjects with single and multiple ascending doses (SAD / MAD) concluded tolerable dose of cellobiose consumption of 20g daily for single-use and 15g twice daily (30g total) for repeated consumption (Annex A: p61-63).
Allergenicity
31. The applicant performed a literature review finding no indication of allergenicity for cellobiose. They also state that the protein content of the NF is below 0.1g/100g and due to the high purity of the product, allergic reactions from protein contamination was highly unlikely.
Committee Action Required
- The Committee is asked whether the available data provide a satisfactory basis for evaluating the safety of this novel food ingredient.
- If so, the Committee is asked whether it is content to recommend approval of the novel food as an ingredient to be added to the range of foods specified.
- If not, the Committee is asked to indicate what additional data would be required.
ACNFP Secretariat
January 2023
Annexes (Confidential)
Annex A – Revised dossier
Annex B – Request for further information (with answers)
Annex C – Supporting document