Joint ACNFP – COT Cannabidiol (CBD) Sub-group Phase 1 Summary Report
On this page
Skip the menu of subheadings on this page.October 2025
Purpose of the sub-group
- CBD ingredients are classified by the FSA as novel foods, meaning any CBD-containing food or supplement must undergo FSA approval prior to legal sale in Great Britain.
- Companies selling CBD-containing foods must submit novel food applications assuring the safety of their products. As per an FSA call for data in February 2020, applicants had to submit their applications and data by 31 March 2021.
- New toxicology studies on CBD ingredients were received by the FSA, and these studies required expert technical review.
- Given the scale of the work anticipated (hundreds of CBD product applicants were expected), a new CBD Working Group was formed as a Sub-group under the ACNFP, to perform the new work and focus on the technical review of new data on CBD ingredients. This group included experts from both the ACNFP and Committee on Toxicity (COT) as below and hence was a Joint Working Group.
Members of the Joint ACNFP-COT CBD Working Group 2022-2025
The Co-Chairs for the duration of the group were the Chair of the ACNFP and toxicologist Dr Camilla Alexander-White, and Chair of the Committee on Toxicity and toxicologist Professor Alan Boobis.
Committee Co-Chairs
Dr Camilla Alexander-White - Chair of the ACNFP
Professor Alan Boobis - Chair of the COT
Committee Members
Ms Alison Austin - ACNFP
Dr Stella Cochrane - COT
Dr James Coulson - COT
Professor Gary Hutchison - COT
Professor Shirley Price - COT
Dr Mac Proven - COT
Dr Cheryl Scudamore - COT
Professor Lesley Stanley - ACNFP
Dr Simon Wilkinson - COT
Secretariat
Ben Haynes - Lead Secretariat for Subgroup
Cath Mulholland - Technical Secretary COT
Olivia Osbourne - COT Secretariat
Priscilla Wanjiru - ACNFP Secretariat
Ruth Willis - Technical Secretariat ACNFP
Tahmina Khan - ACNFP Secretariat
Afielia Choudhry - ACNFP Secretariat
Jenny Rees - ACNFP Secretariat
Victoria Balch - ACNFP and subgroup Administrative Secretariat
Dates of Meetings and Brief Purpose and Outcome of each meeting held
The Joint ACNFP-COT Working Group on CBD met 14 times in the period 2022-2025, on the dates shown in the table below. Final minutes of each meeting can be found at ACNFP and COT Joint Subgroup on CBD and Hemp Derived Products | Advisory Committee on Novel Foods and Processes.
|
1 |
27 July 2022 |
Introductory meeting to share background data and available toxicological evidence for CBD ingredients. NB: It was identified early on in setting up the Working Group that there would be three types of CBD ingredient:
And there would be the issue of assessing the presence and safety of THC as a contaminant, given its status as an illegal drug in GB. |
|
2 |
28 September 2022 |
A first review was performed of new CBD toxicology data on Group A pure CBD isolates (which can be synthesised or extracted from plants) This was based on an initial high level review of the evidence received by the FSA from applicants, and in the knowledge of the COT position on CBD (2020) and an internal FSA CBD literature review performed by the FSA Science team. |
|
3 |
30 November 2022 |
Data from the ACI Consortium on CBD was reviewed in detail – RESERVED BUSINESS. |
|
4 |
17 January 2023 + extended to 13 February 2023 |
Data from the EIHA Consortium was reviewed in detail - – RESERVED BUSINESS. With continuation of meeting 4 in a 90-minute session in February – all available Group A CBD data were reviewed together in a technical meeting, with POD review and potential Acceptable Daily Intakes initially calculated. Three good quality studies for Pure form CBD were available. |
|
5 |
8 March 2023 |
First Draft of a Position Paper for Group A CBD: outlining an approach to derive a provisional ADI for Group A CBD ≥98% pure. Review of literature data on other cannabinoids that could be in Group B mixtures. Data was very limited. |
|
|
March to July 2023 |
Completion of the Position Paper for Group A CBD was done by correspondence with the Sub-group. |
|
6 |
3 May 2023 |
First review of available data for Group B CBD: including toxicology data, composition data and the possibility of new science on receptor-based activity that could be useful. |
|
7 |
4 July 2023 |
Consideration of criteria for assessing Group B CBD in mixtures with other cannabinoids, based on the review of available data for Group B. |
|
|
October 2023 |
Key Milestone: FSA Published new Consumer Advice (Cannabidiol (CBD) | Food Standards Agency) for Group A CBD with a provisional Acceptable Daily Intake for CBD established at 10 mg CBD/day for a healthy 70kg adult. Given data gaps on reproductive and developmental toxicity and immunotoxicity, the sub-group stressed the importance of continuing the recommendation for labelling of CBD products to protect children, pregnant women, those try to conceive and immunocompromised individuals. |
|
8 |
7 November 2023 |
|
|
9 |
1 February 2024 |
A whole day in person/hybrid meeting in London:
|
|
10 |
22 May 2024 |
Review of new additional evidence on Group A CBD – check of the provisional ADI relevancy and status – all good.
|
|
11 |
16 July 2024 |
|
|
12 |
11 September 2024 |
|
|
13 |
6 November 2024 |
|
|
14 |
22 January 2025 |
|
|
|
Q1 2025 |
Key Milestone: Completion of ‘Statement on a THC safe upper limit completed by correspondence. Joint position paper from the Advisory Committee on Novel Foods and Processes (ACNFP) & Committee on Toxicity (COT) on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as contaminants of hemp-derived products including CBD novel foods.’ Harmonised with the work in UK by the Department of Health and Social Care on the misuse of drugs, and with the EFSA Opinion in the EU. Safe Upper Limit defined as 1 µg/kg bw/day based on scientific evidence. |
Understanding the data on CBD
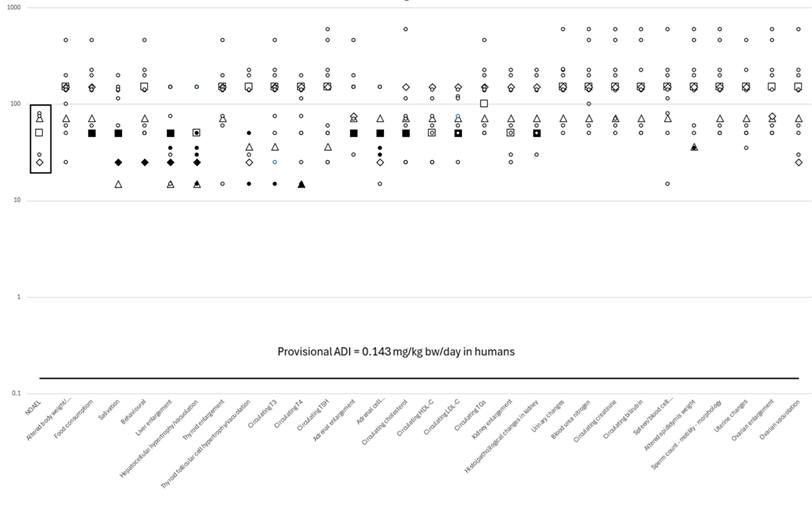
To provide an illustration of the data seen to date figure 1 summarises the sub chronic data submitted to support applications for 98% and above CBD novel foods. The figure identifies the doses at which effects were seen in the study reviewed. The x axis outlines the range of statistically significant effects seen for CBD and the doses at which these were observed.
As noted by the CBD Subgroup, CBD exerts a wide variety of affects and it is impossible to identify a single primary endpoint for use as a Point of Departure for risk assessment. This means that, rather than being driven by a single diagnostic endpoint, establishment of a NOAEL for any study on CBD requires a holistic assessment of all the effects observed. The CBD Subgroup based its advice on a constellation of effects, mainly those in the liver, thyroid, adrenal and kidney. The data indicates that the most sensitive effects are those on liver and thyroid and there is a gap between the effects seen and the provisional ADI to protect the population.
The data on the doses where effects were seen in the sub-chronic study are compared in figure 1 to the level established as the provisional ADI. This shows the margin between the lowest dose at which the effect was seen and the advice of the subgroup. This provides reassurance that consuming 98% purity and above CBD at 10mg a day is unlikely to lead to adverse effects.
Conclusions & Recommendations
The CBD Working Group has delivered the following:
a) A position paper – establishing a science based provisional ADI for Group A CBD ingredients ≥98% pure at 10 mg CBD/day for a healthy 70 kg adult, which has formed the basis of updated FSA Consumer Advice in October 2023. Important caveats remain about data gaps for reproductive toxicity and immunotoxicity leading to advisory statements about the protection of children, pregnant women, those trying to conceive a baby and immunocompromised individuals.
b) A position paper – deriving a safe upper limit of 1 µg/kg bw/day for the contaminant THC in CBD ingredients, based on the scientific evidence, which can be used in setting specifications and in novel foods safety assessment to ensure the safety of consumers from this illegal drug.
c) Clear advice that Group B and Group C CBD ingredients will need to be considered case-by-case by the ACNFP in novel foods applications.
d) An FSA Evidence base to underpin the justification for the Group A provisional ADI, with a useful graphic to explain how the ADI protects against all potential health effects of CBD.
Fig 1: FSA Evidence Base for Group A: A graphical representation of the data from Group A CBD ≥98% pure 90-day studies, including from the three pivotal studies that are used to establish a provisional ADI for Group A CBD ≥98% pure ingredients, Shows the provisional ADI is protective of all observed effects from CBD relevant to humans.