Committee Advice on the safety of Cellobiose as a novel food
On this page
Skip the menu of subheadings on this page.Reference number RP1109
Food Standards Agency (FSA) and Food Standards Scotland (FSS)
Regulated Product Dossier Assessment
Version 1
Assessment finalised: November 2024
Summary
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in May 2021 from Savanna Ingredients GmbH (“the applicant”) for the authorisation of (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol also known as Cellobiose, a sugar replacement ingredient.
This new novel food application is for a disaccharide consisting of two glucose units linked by a β-1-4 glycosidic bond obtained from two-step enzymic conversion of sucrose and glucose into Cellobiose. The applicant intends to use the novel food as an ingredient in various food categories including food supplements, to partially replace sugars such as sucrose or lactose with the function of a low-calorie sweetener. The novel food ingredient and food supplements are not intended to be consumed by those under one year of age (infants).
To support the FSA and FSS in their evaluation of the application, the Advisory Committee on Novel Foods and Processes (ACNFP) were asked to review the safety dossier and supplementary information provided by the applicant. Please note the Committee did not consider any potential health benefits or claims arising from consuming the food, as the focus of the novel food assessment is to ensure the food is safe, and not putting consumers at a nutritional disadvantage.
The Committee concluded that the applicant had provided sufficient information to assure the novel food, Cellobiose, was safe under the conditions of use. The anticipated intake level and the proposed use as a food ingredient was not considered to be nutritionally disadvantageous.
1. Introduction
1. The ACNFP assessed the food safety risks of (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol also known as Cellobiose and its production under the proposed uses, in line with Article 7 of regulation (EU) 2017/2469, retained in UK law. The regulatory framework and the retained technical guidance published by EFSA for full novel food applications (and is applicable to extension of use applications) formed the basis and structure for the assessment (EFSA NDA Panel, 2021).
2. In May 2021 from Savanna Ingredients GmbH (“the applicant”) for the authorisation of (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol also known as Cellobiose, a sugar replacement ingredient. The novel food is a disaccharide consisting of two glucose units linked by a β-1-4 glycosidic bond obtained from two-step enzymic conversion of sucrose and glucose into Cellobiose. The applicant intends to use the novel food as an ingredient in various food categories to partially replace sugars such as sucrose or lactose with the function of a low-calorie sweetener. As the novel food is a disaccharide it is not subject to the food additives legislation.
3. Following the review by the ACNFP in February 2023, further information was requested from the applicant concerning the production process, proposed use levels and ADME on Cellobiose, in order to address information gaps in the initial dossier. The final advice from the Committee was agreed at the 163rd meeting, allowing the FSA and FSS to complete the risk assessment.
4. This Committee Advice Document (CAD) outlines the conclusions of the ACNFP on the safety of Cellobiose as a novel food.
2. Assessment
2.1 Identity of novel food
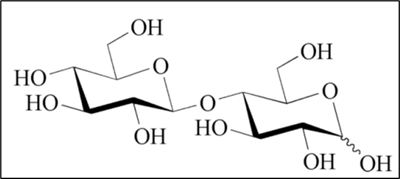
5. The novel food, Cellobiose, is classified as glucose monomers linked through a β1,4-glycosidic bond (Figure 1) and characterised by the following information:
IUPAC name (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol
CAS number 528-50-7
Molecular weight 342.3 g/mol
Molecular formula C12H22O11
Figure 1- Molecular structure of Cellobiose.
6. The identity was verified by Nuclear Magnetic Resonance (NMR) analyses confirming the beta-glycosidic bond between the two glucose moieties that is characteristic for Cellobiose. Molecular mass of the novel food was analysed by mass spectrometry.
7. The identity of the novel food was considered to be appropriately characterised.
2.2 Production Process
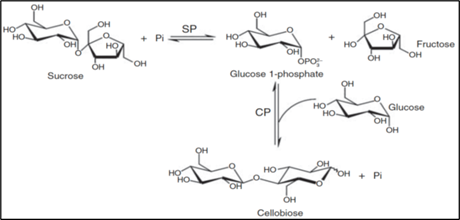
8. Cellobiose is catalysed by a two-step enzymatic reaction converting sucrose and glucose to Cellobiose, with the release of fructose. The general principle of the enzymic reaction was described by Brucher and Häßler (Brucher and Häßler, 2019), with the schematic depiction of the conversion shown in Figure 2.
Figure 2. Enzymatic conversion of glucose and sucrose into Cellobiose and fructose. SP=sucrose phosphorylase;CP = CellobiosePhosphorylase (from Brucher and Häßler (Brucher and Häßler, 2019).
9. The enzymes involved are sucrose phosphorylase (SP) and Cellobiose phosphorylase (CP) where the first converts sucrose into fructose and glucose 1-phosphate (G1P), which is a substrate of Cellobiose Phosphorylase that synthesizes Cellobiose by coupling one molecule of glucose to one molecule of G1P via a ß-1-4 glycosidic bond.
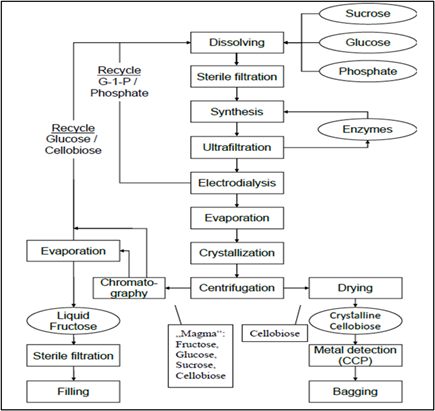
10. After conversion, Cellobiose is separated from the enzymes by ultrafiltration, then purified by electrodialysis. The liquor collected is concentrated by evaporation and the final product is obtained from the concentrated liquor by crystallization. A manufacturing schematic flow chart has been provided in Figure 3.
Figure 3. Manufacturing flow scheme for Cellobiose. G-1-P is glucose-1-phosphate; Critical Control Point (CCP) is a sifter with metal detection.
11. Based upon the advice of the ACNFP, further information was sought on the purity of the enzymes and the parameters used to ensure the reaction was effectively managed to minimise variability and unsure a consistent product was produced. It was noted that the two enzymes used, sucrose phosphorylase and Cellobiose phosphorylase, are both under application with EFSA by their manufacturers and provided their specifications.
12. To appropriately control the enzymatic reactions the activity of each incoming enzyme is tested. On this basis additional purity criteria within the production process were not considered to be required. Information was reviewed on the efficiency of the enzyme’s activity and the basis for the controls within the process.
13. The ACNFP concluded that the production process was sufficiently characterised. Key areas of food safety hazards had been identified and effectively managed. It was noted that the enzymes in the process were being subject to a separate evaluation in the EU. A parallel evaluation has yet to begin in GB. As a result, particular focus was given to the potential for active enzyme to be present in the final product. The ACNFP considered the processes in place had addressed the potential risk.
2.3 Compositional Information
14. Results from five independently manufactured batches of Cellobiose were provided (Table 1). Analysis was performed by accredited laboratories with certifications provided. These demonstrate the characteristics of the novel food and the effective management of any hazards identified.
Table 1. Analyses of five independent batches of Cellobiose.
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
Appearance |
HH-MA-M 10-016: 2002-05 |
--- |
White Powder |
Complies |
Complies |
Complies |
Complies |
Complies |
--- |
--- |
|
Smell |
HH-MA-M 10-016: 2002-05 |
--- |
Typical |
Complies |
Complies |
Complies |
Complies |
Complies |
--- |
--- |
|
Taste |
HH-MA-M 10-016: 2002-05 |
--- |
Typical |
Complies |
Complies |
Complies |
Complies |
Complies |
--- |
--- |
Identity
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
NMR Spectra |
1H-NMR |
--- |
Typical |
Complies |
Complies |
Complies |
Complies |
Complies |
--- |
--- |
Physico-chemical parameters
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
Refraction index |
Ph. Eur. 2.2.6: 2008-01 |
°Brix |
--- |
1.348 |
1.348 |
1.349 |
1.349 |
1.348 |
1.35 |
0.00 |
|
Optical rotation |
Ph. Eur. 10.0/0188 Fructose (20 °C, c=10 %) |
° |
33-36 |
35.1 |
34.8 |
33.9 |
34.4 |
35.1 |
34.66 |
0.46 |
|
rel. Density |
Ph. Eur. 2.2.5: 2008-01 |
kg/L |
--- |
1.0389 |
1.0393 |
1.0403 |
1.0415 |
1.0391 |
1.04 |
0.00 |
|
pH |
Ph. Eur. 2.2.3: 2016-07 |
[-] |
--- |
5.4 |
4.2 |
3.7 |
3.7 |
4.3 |
4.26 |
0.62 |
|
Melting point |
Ph. Eur. 2.2.15, mod. |
°C |
235-241 |
237.4 |
236.6 |
234.6 |
235.1 |
237.5 |
236.24 |
1.19 |
|
Dry matter |
§ 64 LFGB L 39.00-2(EG): 1981-04 |
g/100 g |
≥ 99 |
≥ 99 |
≥ 99 |
≥ 99 |
≥ 99 |
≥ 99 |
--- |
--- |
|
Water activity |
Application Rotronic für AwTherm (2015-11) |
[-] |
--- |
0.495 |
0.491 |
0.463 |
0.436 |
0.487 |
0.47 |
0.02 |
Chemical analysis
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
Cellobiose |
§ 64 LFGB L 40.00-7/KIN CH 013 |
g/100 g |
≥ 99 |
99.4 |
99.5 |
99.7 |
99.3 |
99.8 |
99.54 |
0.19 |
|
Fructose |
§ 64 LFGB L 26.11.03-8: 1983-05 |
g/100 g |
in sum ≤ 1 |
0.19 |
0.18 |
0.28 |
0.54 |
0.21 |
0.28 |
0.13 |
|
Glucose |
§ 64 LFGB L 26.11.03-8: 1983-05 |
g/100 g |
in sum ≤ 1 |
0.13 |
0.11 |
0.24 |
0.26 |
0.36 |
0.22 |
0.09 |
|
0.12Sucrose |
§ 64 LFGB L 26.11.03-8: 1983-05 |
g/100 g |
in sum ≤ 1 |
< 0.10 |
< 0.10 |
< 0.10 |
0.12 |
0.21 |
0.17 |
0.05 |
|
Maltose |
§ 64 LFGB L 26.11.03-8: 1983-05 |
g/100 g |
--- |
< 0.1 |
< 0.1 |
< 0.1 |
< 0.1 |
< 0.1 |
< 0.1 |
--- |
|
Ash |
§ 64 LFGB L 17.00-3: 1982-05 |
g/100 g |
≤ 0.1 |
< 0.10 |
< 0.10 |
< 0.10 |
< 0.10 |
< 0.10 |
--- |
--- |
|
Ash, HCl insoluble |
§ 64 LFGB L 53.00-4: 1996-02 |
g/100 g |
--- |
< 0.10 |
< 0.10 |
< 0.10 |
< 0.10 |
< 0.10 |
--- |
--- |
|
Phosphate |
DIN EN ISO 6878 (D11): 2004-09 |
mg/kg d.m. |
--- |
55 |
24 |
25 |
20 |
31 |
--- |
--- |
|
Protein |
Bradford (Roth NanoQuant®) |
mg/100 g |
< 10 |
3.1 |
2.5 |
2.6 |
2.9 |
3,1 |
2.9 |
0.4 |
Microbial Analysis
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
Total aerobic count |
DIN EN ISO 4833-1: 2013-12 |
cfu/g |
≤ 1000 |
< 10 |
< 10 |
< 10 |
160 |
< 10 |
--- |
--- |
|
Yeast |
§ 64 LFGB L 01.00-37: 1991-12 |
cfu/g |
≤ 100 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
--- |
--- |
|
Mould |
§ 64 LFGB L 01.00-37: 1991-12 |
cfu/g |
≤ 100 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
--- |
--- |
|
Enterobacteriaceae |
DIN EN ISO 21528-2: 2017-09 |
cfu/g |
--- |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
--- |
--- |
|
Coliforms |
ISO 4832: 2006-02 |
cfu/g |
≤ 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
--- |
--- |
|
Staphylococcus aureus, coag. pos. |
DIN EN ISO 6888-1: 2003-12 |
cfu/g |
--- |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
--- |
--- |
|
Salmonella |
§ 64 LFGB L 00.00-20: 2018-03 |
/25 g |
negative |
negative |
negative |
negative |
negative |
negative |
--- |
--- |
|
E. coli |
ISO 7251: 2005-02 |
/10 g |
negative |
negative |
negative |
negative |
negative |
negative |
--- |
--- |
|
E. coli |
VA10-009-V07 |
/10 g |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
--- |
--- |
Heavy Metals
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
Lead |
DIN EN 15763, ICP-MS: 2010-04 |
mg/kg |
< 0.05 |
< 0.020 |
< 0.020 |
< 0.020 |
< 0.020 |
< 0.020 |
--- |
--- |
|
Cadmium |
DIN EN 15763, ICP-MS: 2010-04 |
mg/kg |
< 0.01 |
< 0.010 |
< 0.010 |
< 0.010 |
< 0.010 |
< 0.010 |
--- |
--- |
|
Mercury |
DIN EN 15763, ICP-MS: 2010-04 |
mg/kg |
< 0.01 |
< 0.010 |
< 0.010 |
< 0.010 |
< 0.010 |
< 0.010 |
--- |
--- |
|
Arsenic |
DIN EN 15763, ICP-MS: 2010-04 |
mg/kg |
< 0.1 |
< 0.040 |
< 0.040 |
< 0.040 |
< 0.040 |
< 0.040 |
--- |
--- |
Residual Genomic DNA
|
Parameter |
Test method |
Units |
Specification |
L1018025 |
L1018031 |
L1018205 |
L1018231 |
L1017514 |
mean |
Stdev
|
|
Recombinant DNA |
c-LEcta SOP-QC-69 v1.0 |
ppm |
< 0.01 ppm |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
--- |
--- |
|
Recombinant DNA |
c-LEcta SOP-QC-70 v1.0 |
ppm |
< 0.01 ppm |
n. d. |
n. d. |
n. d. |
n. d. |
n. d. |
--- |
--- |
15. The protein content for Cellobiose was analysed internally using the ready-to-use ROTI® Nanoquant assay. The limit of detection (LOD) for the method was determined to be 1.1mg per 100g and the Limit of Quantitation (LOQ) 3.4mg per 100g.
16. Two recombinant enzymes are used in the enzymic production of Cellobiose. A test to determine the absence of recombinant DNA (rDNA) derived from the production strains was undertaken for 5 Cellobiose batches with results showing no rDNA was detected.
17. The compositional information did not raise further specific questions.
2.4 Stability
18. Stability studies for Cellobiose were conducted in normal storage conditions at 25°C and 60% relative humidity (RH) and accelerated conditions of 40°C and 75% relative humidity with the key parameters tested being microbial, chemical and sensory for a period of 24 months.
19. The novel food was stable for at least 24 months in normal conditions (25°C/60 % RH) and at least 6 months in accelerated conditions (40°C/75 % RH), with glucose concentrations remaining below 1%. No major colour changes were reported during the course of the study.
20. The water activity remained constant under normal conditions and increased from 0.47 to 0.53 at accelerated conditions. The levels were considered low enough to limit microbial growth under both storage conditions.
21. The ACNFP considered the information provided to sufficiently demonstrate stability of the novel food for up to 24 months.
2.5 Specification
22. The specification parameters for the novel food were provided (Table 2). No other sugars besides Cellobiose are expected in the product. Cellobiose and glucose were deemed to be suitable parameters to characterize and substantiate the purity of the novel product.
Table 2. Specifications of Cellobiose.
Sensory Characteristics.
|
Parameter |
Specification |
Method |
|
Appearance |
White powder |
HH-MA-M 10-016: 2002-05 |
|
Odour and Taste |
Product typical |
HH-MA-M 10-016: 2002-05 |
General Characteristic
|
Parameter |
Specification |
Method |
|
Dry substance % |
≥ 99 |
§64 LFGB L 39.00-2(EG): 1981-04 |
|
Cellobiose % |
≥ 99 |
§64 LFGB, L 40.00-7; VA KIN CH 013 |
|
Other identified sugars % |
≤ 1 |
§64 LFGB L 26.11.03-8: 1983-05 |
|
Optical rotation (°mL/dm g) |
33-36 |
Ph. Eur. 10.0/0188 Fructose |
|
Ash g/100g |
< 0.1 |
§ 64 LFGB L 17.00-3: 1982-05 |
|
Melting point °C |
235-241 |
Ph. Eur. 2.2.15 |
|
Protein content mg/100g |
< 10 |
Bradford Assay |
Heavy metals
|
Parameter |
Specification |
Method |
|
Arsenic mg/kg OS |
< 0.1 |
DIN EN 15763, ICP-MS: 2010-04 |
|
Mercury mg/kg OS |
< 0.05 |
DIN EN 15763, ICP-MS: 2010-04 |
|
Lead mg/kg OS |
< 0.01 |
DIN EN 15763, ICP-MS: 2010-04 |
|
Cadmium mg/kg OS |
< 0.01 |
DIN EN 15763, ICP-MS: 2010-04 |
Microbiology
|
Parameter |
Specification |
Method |
|
Total aerobic count cfu/1g |
≤ 1000 |
DIN EN ISO 4833-1: 2013-12 |
|
Yeast cfu/1g |
≤ 100 |
§64 LFGB L 01.00-37: 1991-12 |
|
Mould cfu/1g |
≤ 100 |
§64 LFGB L 01.00-37: 1991-12 |
|
Salmonella cfu/25g |
Negative |
§64 LFGB L 00.00-20: 2018-03 |
|
Coliforms cfu/1g |
< 10 |
ISO 7251: 2005-02 |
|
E. coli cfu/10g |
Negative |
ISO 7251: 2005-02 |
|
Storage conditions |
|
Store at room temperature and away from light |
23. Table 1 in the section above provides information for 5 batches of the novel food and compares these to the specification. The results demonstrate the production of the novel food is reproducible and consistently produces the novel food within the specification, at commercial scale.
24. The information provided is sufficient for the specification of Cellobiose, and appropriately characterises the novel food seeking authorisation.
2.6 History of Use
25. Sucrose and glucose have a long history of safe use as a food ingredient and are present in foods such as vegetables, fruits and other sugar-rich foods such as honey or sugar cane. Pure Cellobiose has not been used as a food ingredient before and therefore no information on its previous use was provided.
26. Literature review carried out found that honey and developing maize grains have trace amounts of Cellobiose present (Gentinetta et al., 1979). In honey Cellobiose is present at levels of 0.06–0.28 g/100g (Sanz et al., 2004; de la Fuente et al., 2006) and developing maize grains have Cellobiose present at levels of up to 0.05 mg/g in the embryo and 0.06-0.13 mg/g in the endosperm.
27. Minor exposure for consumers from other sources of Cellobiose was noted but was not considered to provide a significant contribution to the exposure of Cellobiose if the novel food was authorised.
28. The history of use does not indicate any further areas for evaluation.
2.7 Proposed Use and Anticipated Intake
29. Cellobiose is proposed to be used as a food supplement by the general population excluding those under one year of age at a maximum level of 60,000mg per 100g. For food supplements, the target population is the general population (excluding those under one year of age) at a maximum level of 80,000mg per 100g. The applicant has also requested for the novel food to be used as an ingredient in several categories to replace sucrose or lactose, or function as a low calories’ sweetener.
30. An assessment was made of the exposure to the novel food from the proposed uses using the FAIM tool. The tool included UK data at the time of the calculation. This assessment provided a basis to refine the food categories and proposed use levels. The applicant then compared the potential exposure to the novel food to tolerable upper intakes identified by age group from the human studies. A maximum overall daily intake of cellobiose was identified as 0.44 g/kg bw/day. The single doses maximum intake was identified as not more than 0.26 g/kg bw per day (More et al., 2019) or 0.29g/kg bw/day once corrected for a 70kg adult. Table 3 outlines the maximum intake stratified by population group. For toddlers and young children, the value was derived from a study for adults and as such it was noted that it should be interpreted with caution for children.
Table 3. Proposed maximum intake of Cellobiose derived from the human tolerance study.
|
Population Group |
Age |
Bw [kg] |
Proposed maximum daily intake [g] - single dose 0.26 g/kg bw/day |
Proposed maximum daily intake [g] - overall daily intake 0.44 g/kg bw/day |
|
Toddlers |
12-35 m |
11.9 |
3 |
5 |
|
Other children |
36 m - 9 y |
23.1 |
6 |
10 |
|
Adolescents |
10 y – 17 y |
50.0 |
13 |
22 |
|
Adults |
18 y – 64 y |
73.9 |
19 |
32 |
|
Elderly and very elderly |
> 65 y |
75.0 |
19.5 |
33 |
31. When the calculation of exposure is compared to the safe upper intake identified, the lower- and upper-bound mean intake by all subjects and the lower-bound intake of the 95th percentile on consuming days for adolescents, adults and the elderly are below the tolerable consumption level of 0.26 g/kg bw/day (with one serving as established in the human tolerance study (More et al., 2019) before correction to 70kg adult).
32. The upper-bound intake levels in the 95th percentile by adolescents on consuming days slightly exceeds the highest tolerable Cellobiose level of 0.44 g/kg bw/day in two servings. This was not considered a concern as the limitations of the approach used would suggest the outcome from the calculations will be an overestimate of consumption.
33. Consideration was also given to other sources of cellobiose in the diet. As outlined in the history of use section other natural sources of Cellobiose are present at low levels in some commonly consumed food. It is not expected for these sources to significantly contribute to Cellobiose intake hence the mean and high daily intake proposed are representative for total intake of the novel food from all sources.
34. Following review of the original exposure assessment the applicant amended their proposed uses as described in Table 4. This was the basis for the review going forward.
Table 4: Updated food categories (FoodEx2) and maximum intended use levels for Cellobiose [mg/100 g].
|
Food Category |
Maximum intended use (mg/100g) |
|
Animal meat dried |
2,000 |
|
Canned-tinned meat |
2,000 |
|
Raw cured (or seasoned) meat |
2,000 |
|
Cooked cured (or seasoned) meat |
2,000 |
|
Fresh raw sausages |
2,000 |
|
Meat based spreadable-textured specialties |
2,000 |
|
Liver based spreadable-textured specialties |
2,000 |
|
Special food for children's growth |
1,000 |
|
Meat imitates |
2,000 |
|
Preserved or partly preserved sausages |
2,000 |
|
Savoury sauce dry preparation |
40,000 |
|
Table-top sweeteners in powder form |
60,000 |
|
Table-top sweeteners in tablets |
60,000 |
|
Food Supplements and similar preparations |
80,000.00 (max 3g /day) |
35. The exposure calculation was recalculated for the revised proposed uses using the DietEx tool which cumulates chronic intake data from all participating members on all reporting days and consuming days. The data was compared to the human tolerance study where an amended value of 1 x 20g Cellobiose (0.29 g/kg bw/day once corrected for a 70kg adult) or 2 x 15 g Cellobiose (30g in total, corresponding to 0.44 g/kg bw/day) was identified as being well tolerated in healthy human subjects.
36. The recalculated exposure suggests that excluding food supplements use, the resulting anticipated intake in the 95th percentile by all age groups in the whole population is below the tolerable level for a single dose of 0.29g/kg bw/day, once corrected for a 70kg adult, that was determined in a human tolerance study (More et al., 2019) (Table 5).
Table 5: Anticipated intake of Cellobiose according to the proposed uses and use levels listed in Table 3 calculated with DietEx (version of March 30, 2023), excluding food supplements.
|
Population group |
Min mean [mg/kg bw/day] |
Max mean [mg/kg bw/day] |
Min 95th [mg/kg bw/day] |
Max 95th [mg/kg bw/day] |
Min mean [mg/day] |
Max mean [mg/day] |
Min 95th [mg/day] |
Max 95th [mg/day] |
|
Toddlers |
4.39 |
48.70 |
23.26 |
137.17 |
52.02 |
599.28 |
260.00 |
1.800.00 |
|
Other children |
3.24 |
45.30 |
15.63 |
127.27 |
69.39 |
981.97 |
333.33 |
2.435.00 |
|
Adolescents |
6.47 |
25.67 |
20.96 |
79.23 |
368.83 |
1.224.16 |
1.111.90 |
3.900.00 |
|
Adults |
4.18 |
21.00 |
16.82 |
65.20 |
309.77 |
1.650.51 |
1.300.00 |
5.155.00 |
|
Elderly |
1.89 |
17.85 |
11.79 |
57.06 |
145.92 |
1.413.94 |
950.00 |
4.500.00 |
|
Very elderly |
2.52 |
15.28 |
11.28 |
42.74 |
184.80 |
1.153.44 |
879.52 |
4.209.09 |
|
Pregnant women |
3.54 |
7.24 |
14.74 |
32.04 |
229.47 |
502.09 |
900.00 |
2.360.00 |
|
Lactating women |
1.70 |
9.72 |
6.93 |
35.93 |
114.56 |
620.84 |
443.33 |
2.200.00 |
|
Vegetarians |
3.14 |
3.14 |
24.53 |
24.53 |
194.67 |
194.67 |
1.300.00 |
1.300.00 |
37. In interpreting the data, it was noted that it was not expected that consumers would be consuming both food supplements and the other food categories. A calculation was made to understand the impact of consuming all food categories. It was noted that in the theoretical case that a consumer in the highest percentile would take a food supplement providing 3 g cellobiose per day (excluding infants and toddlers), the tolerance levels for the respective age groups would not be exceeded.
38. Queries were raised on the use levels of Cellobiose in the proposed food categories listed according to the lower-bound intake of the 95th percentile of 0.26 g/kg bw/day (with one serving as established in the human tolerance study) and the upper-bound intake levels in the 95th percentile by of 0.4 g/kg bw/day in two servings given the potential for diarrhoea as one of the potential adverse effects. Further consideration was warranted to understand the likely pattern of exposure.
39. Further analysis was undertaken to understand the impact of a number of lower dose intakes simultaneously rather than cumulative exposure. To address this, a mock diet/meal-based approach was developed to better illustrate a worst-case scenario.
40. The applicant calculated exposure that was tailored for each age group to take account of differences in portion size and consumption of some food categories. The analysis used a mock diet to explore the impact, based on food consumption data from DietEx at the 95% consumers with all proposed uses being consumed at a single eating occasion.
41. The data indicated that for adults, adolescents and those under 1 year of age the maximum intake/tolerance level for a single eating occasion for each age group identified from the human study would not be exceeded. For the 4–10-year-olds, and 1–3-year-olds the tolerance level would be exceeded slightly at 9.3g respectively and 4.9g respectively, when compared to the level considered tolerable at a single eating occasion for these age groups of 5.9g and 3.0g. However, in interpreting this information it was noted the limitations in modelling potential exposure for these groups given the information available on eating habits, as well as that to consume the full range of products in the mock diet in a single sitting would represent an unlikely scenario. As such the estimates are considered to be conservative. If the proposed uses were consumed over 2 or more eating occasions the tolerance level for the respective ages would not be exceeded.
42. Potential impact on vulnerable populations including whether there would be an impact on those with diabetes was also considered. It was noted that Nakamura et al (2004) had shown that 25g of cellobiose did not increase blood sugar or insulin secretion in contrast to glucose. As such it was not expected that there were specific risks for this population for risk managers to be aware of.
43. The information presented was considered and it was concluded exposure from the revised proposed uses and use levels would not exceed the level identified as safe from the human tolerance study. As such, it was concluded there were no concerns with the proposed uses and use level.
2.8 Absorption, Distribution, Metabolism and Excretion (ADME)
44. A systematic literature review on Cellobiose in humans provided evidence that Cellobiose is not absorbed in significant quantities in the human intestine of healthy individuals (Nakamura et al., 2004). The data suggested digestibility of Cellobiose might be different in populations with different lactase activities. The authors suggested that Cellobiose is slowly hydrolysed by lactase, but not by other intestinal enzymes. No evidence was presented on the impact of populations with increased or decreased gut permeability. It was also noted that the data presented (Cobden et al., 1982 and Hamilton et al., 1982) suggested Cellobiose was not absorbed in the intestine in significant quantities but increased significantly during episodes of active coeliac disease (Cobden et al, 1980 and Hamilton et al., 1982). Absorption of cellobiose is considered a marker for mucosal damage and intestinal mal absorption in Coeliac disease.
45. Literature also reported no effect on blood sugar or insulin secretion. Nakamura et al., (2004) investigated the effects of Cellobiose and glucose on blood sugar, insulin secretion and breath hydrogen in ten healthy young women. 25g of Cellobiose ingested did not increase blood sugar or insulin secretion in contrast of glucose. Thus, the authors concluded that cellobiose is well fermented in the human large intestine and its available energy content was estimated to be 2 kcal/g (Nakamura et al., 2004).
46. To further understand the ADME properties of the novel ingredient, experiments to investigate resistance of Cellobiose to brush border cell-derived enzymes and to intestinal absorption had been provided. These showed that the novel food was not significantly degraded by the enzymes of these cells. Following the review, it was noted that the pH study while providing initial results that Cellobiose was not broken down in gastric conditions, did not simulate digestion and in particular the potential for fermentation of Cellobiose in the gut (Unpublished report, 2013).
47. A human study investigating glycaemic and insulinemic indices following the ingestion of cellobiose or allulose, in a monocentric, randomised, cross-over, simple blind, referent-controlled human clinical trial was carried out by the applicant. The study involved fourteen healthy adults where glucose, Cellobiose or allulose were administered at five different visits each 48 hours apart (Unpublished report, 2021).
48. The results confirmed Cellobiose is not absorbed nor digested to a significant extent in the small intestine but metabolized by the colonic microbiota. The evidence presented was found to be consistent with human data from a study by Nakamura et al., (2004). This data, along with the information that maltose but not Cellobiose is not significantly hydrolysed at the brush border, supports the hypothesis that it is not absorbed and remains available for metabolism by intestinal microflora.
49. All the information provided by the applicant was considered and it was concluded that sufficient evidence had been presented to suggest Cellobiose is not hydrolysed in the gut or GI tract until metabolised by colonic microflora. As such it is unlikely to be absorbed. This was considered as evidence that it would not pose additional risks for diabetics as a subpopulation.
2.9 Nutritional information
50. The literature on the nutrition of the novel food indicates an energy value could be calculated from the available data of 2kcal/g (Nakamura et al., 2004, Gill et al., 2006, Cantarel et al., 2012, Zanten et al., 2012, Andersen et al., 2012, Andersen et al., 2013, Yan et al., 2017, Mika et al., 2016, Ilhan et al., 2017, Magnúsdóttir et al., 2017). The energy value was derived from the value of 2kcal/g of Cellobiose published in literature.
51. Experiments to investigate the prebiotic properties of Cellobiose with tests on stability of Cellobiose in gastric pH conditions were undertaken. The results indicated that there was no release of reducing sugars or glucose from Cellobiose or inulin during digestion conditions. Starch was hydrolysed in the testing conditions.
52. Based on the data presented the novel food is intended to replace other sugars in the diet. As such, consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
2.10 Toxicological information
2.10.1 Genotoxicity
53. The novel food was tested for mutagenicity in a reverse mutation assay using Salmonella typhimurium (TA98, TA100, TA1535 and TA1537 and in one Escherichia coli strain WP2 uvrA [pKM101]. The assay was performed with doses at 31.6, 100, 316, 1000, 3160 and 5000 µg Cellobiose/plate with and without activation using a liver preparation. The test was Good Laboratory Practice (GLP) compliant and in line with OECD Test Guideline (TG) 471. No increase in revertant colony numbers as compared with control counts was observed for Cellobiose indicating the novel food was not mutagenic (Unpublished data, 2017a).
54. A GLP-compliant in vitro micronucleus test on the novel food, using peripheral human lymphocytes was conducted in line with OECD TG 487. The test was conducted with and without the presence of activation (S9 mix). Concentrations of 3.16, 10.0, 31.6, 100, 316, 1000 and 2000 µg Cellobiose/mL were tested with incubations of 4 or 24 hours with activation and 4 hours without activation. No dose-related increase in micronuclei compared to controls were seen up to the top concentration of 2000 µg/mL. The frequency of micronucleated cells was within the historical control range. It was concluded that the novel food was not clastogenic (Unpublished data, 2017b).
2.10.2 Sub-chronic Toxicity
55. Two studies on the novel food were provided. A 28-day dose range finding study administered to 12 male and 12 female rats in 4 groups using +0%, 5%, 10%, 15% of the novel food in the drinking water (Unpublished report, 2017c; Winkler et al., 2020). This resulted in exposures of 3.95g-8.40g/kg/bw/day. Males in the 10% and 15% NF dose groups and female in the 15% NF dose group reported slightly reduced body weight linked to a reduction in water and food consumption. This data was used to inform the design of the 90-day study.
56. A 90-day study was undertaken using the OECD 408 test guideline using 50 male and 50 female rats in 4 groups at doses of 0%, 2.5%, 5%, 10% of the novel food dosed in drinking water as a vehicle. The results of the study also indicated that males in the 5% and 10% Cellobiose dose groups had reduced body weight and a decrease of food consumption. The experimental no-observed-adverse-effect level (NOAEL) was ≥10% Cellobiose in drinking water ~ 6852 mg and ~ 8043 mg/kg bw/day for males and females respectively. All the observed effects (marginally reduced body weight and food consumption) were considered to be secondary to a reduced drinking water intake at the highest dose level (Unpublished report, 2017d; Winkler et al., 2020).
57. A systematic literature review on the toxicity of Cellobiose was undertaken. The results reported in Moinuddin and Lee (1958) suggest that at 15% cellobiose in the diet, gastric effects such as diarrhoea may be seen but decrease in severity over time. This study also indicated impacts on body weight, and relative organ weight at this dose. The pattern of effects is consistent with those seen in the sub-acute and subchronic studies (Unpublished report, 2017 c and d) on the novel food.
58. Following work on other sugars such as Allulose, (German Bundesinstitut für Risikobewertung (BfR), 2020) the potential for Cellobiose to impact the virulence of K. pneumoniae was investigated (Wu et al., 2012). From the data available, while deletion of a gene for Cellobiose uptake decreased the virulence of K. pneumoniae under low carbon source conditions, there was no evidence to suggest Cellobiose consumption would increase the virulence of K. pneumoniae.
59. Additionally, Cellobiose has been used as clinical marker for intestinal permeability in various studies (Cobden et al., 1980). It has proven to be an effective marker for mucosal damage and intestinal malabsorption in Coeliac disease (Cobden et al., 1985, Hamilton et al., 1982). This use of cellobiose has been without adverse effects in the consumers supporting the safety of the substance.
2.10.3 Human Studies
60. A human tolerance study (More et al., 2019) was performed in healthy subjects using cellobiose in two phases. The first, a Single Ascending Dose (SAD) study where doses of 10, 15, 20 and 25g of cellobiose were administered to 6 subjects per dose. This SAD study concluded the tolerable dose of Cellobiose consumption of 20g (equivalent to 0.26g/kg/bw/day) daily for single use based on the number of participants that experienced gastric effects primarily flatulence and audible stomach noise at the higher dose. Once corrected for a 70kg adult this represents a tolerable intake of 0.29g/kg/bw/day.
61. A second phase was performed using a Multiple Ascending Dose (MAD), where doses of either 15 or 20g of cellobiose were administered twice daily. Again flatulence, stomach noise and impact on stool consistency informed selection of the tolerable dose. This was identified as 15g twice daily (30g total) equivalent to 0.44g/kg/bw/day for repeated consumption (Moré et al., 2019).
62. The data was compared to information from literature. Zanten et al, 2014 undertook a human study of 18 participants, where Cellobiose was given at doses of 15 or 40g per day along with L.acidophilis. Impacts on gut microflora were explored with no change seen in microbial diversity.
63. Nakamura et al 2004, considered the impact of cellobiose consumption on blood sugar, insulin and hydrogen breath levels. 10 healthy participants received 25g cellobiose with no effect seen on blood sugar and insulin response. The changes in breath hydrogen levels were interpreted to support suggestions that cellobiose is fermented in the lower intestine.
2.10.4 Conclusions on Toxicity
64. Following review of the data provided, it was concluded that the data from human studies suggests a maximum safe intake of 20g as a single dose equivalent to 0.26mg/kg/bw and two exposures of 15g (30g in total) equivalent to 0.44 g/kg bw/day as multiple doses (More et al., 2019). This level was based on the human tolerance study of Cellobiose. Once corrected for a 70kg adult this represents a tolerable intake of 0.29g/kg/bw/day. This value has been used for comparisons in the assessment conclusions.
2.11 Allergenicity
65. A literature review was undertaken to identify any data on the allergenicity of Cellobiose. This concluded there was not reported indication of allergenicity for Cellobiose.
66. It was noted that the protein content of the NF as set in the specification is below 10mg/100g. Due to the high purity of the product, allergic reactions to Cellobiose was highly unlikely.
67. To assure that the enzyme does not present a risk for food allergic reactions, information was provided on the steps in the production process which are intended to ensure the enzyme is not present in the novel food. This process was shown to be effective through the low protein content reported for the product. The limitations of the Bradford protein assay employed for detection of protein fragments was explored. It was noted that while more sensitive assays are available, these are confounded by reducing sugars such as the novel food and so the Bradford has been selected.
68. The potential for allergenicity from the enzymes used in the production process of the novel food was highlighted. Further data was supplied that showed the enzyme used had been assessed by EFSA and no specific safety concerns were identified. The feedstock for the production of the enzymes does not contain known allergens and therefore the potential for contamination of the novel food by this route is unlikely.
69. The potential for the enzyme to elicit allergic reactions was explored. A bioinformatics search for the amino acid sequence of the enzyme was undertaken with FASTA alignment using AllergenOnline, Allermatch, and utmbHealth databases and a sliding window of 80 amino acids used. No matches of greater than 35% identity with known allergens were identified.
70. On the basis of the data provided, it was concluded that the novel food was unlikely to elicit allergic reactions in sensitive individuals.
3. Discussion
71. The novel food in this application is Cellobiose, a sugar replacement ingredient. The novel food is a disaccharide consisting of two glucose units linked by a β-1-4 glycosidic bond obtained from two-step enzymic conversion of sucrose and glucose into Cellobiose.
72. The identity was verified through NMR analyses confirming the beta-glycosidic bond between the two glucose moieties that is characteristic for Cellobiose.
73. The applicant intends to use the novel food as an ingredient in various food categories to partially replace sugars such as lactose or sucrose. The target population is the general population excluding those under 1 year of age. Food supplements use excludes infants (those under 1 year of age).
74. An exposure calculation was made using DietEx. The recalculated exposure suggests that excluding food supplements use, the resulting anticipated intake in the 95th percentile by all age groups in the whole population is below the tolerable level for a single dose of 0.29g/kg bw/day that was determined in a human tolerance study.
75. Analysis was also undertaken on the impact of eating multiple food categories in a single eating occasion. This was calculated using a mock diet approach. The results indicate for 4-10 years old and 1–3-year-olds that if all the uses were consumed in a single occasion the tolerable level for a single dose for the respective age group would be exceeded. However, this was thought to be an unlikely scenario, and no concerns were seen when the intake was consumed over 2 or more eating occasions.
76. The toxicological studies conducted by the applicant demonstrated no concerns regarding genotoxicity. The novel food is not considered mutagenic or aneugenic, or clastogenic.
77. Repeated 90-day oral toxicity study in rodents concluded there were no adverse effects with concentrations of 2.5%, 5% and 10% of Cellobiose in drinking water.
78. The single and multiple ascending doses (SAD/MAD) method in the human studies concluded tolerable dose of Cellobiose consumption of 20g daily (0.29 g/kg bw/day for a 70kg adult) for single-use and 15g twice daily (30g total) (0.44 g/kg bw/day) for repeated consumption was identified as being well tolerated. This data was stratified for the population groups under review.
79. Evaluation was made of the allergic potential of the novel food. This included the enzymes used in the production. Based on the residue protein levels and the analysis of the homology of the enzyme amino acid sequence to known allergens, the potential to elicit reactions in sensitive individuals was thought to be unlikely.
4. Conclusions
80. The ACNFP have undertaken the assessment of Cellobiose and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake level and the proposed use in food supplements was not considered to be nutritionally disadvantageous.
81. These conclusions are based on the information in the applicant’s dossier, supplemented by additional information the applicant provided and could not have been reached without the data claimed as proprietary by the applicant.
82. With thanks to the members of the ACNFP during the course of the assessment who were; Dr Camilla Alexander White, Dr Anton Alldrick, Dr Kimon Andreas Karatzas, Ms Alison Austin, Professor George Bassel, Dr Mark Berry, Dr Christine Bosch, Professor Dimitris Charalampopoulos, Dr Catharina Edwards, Professor Susan Fairweather-Tait, Professor Paul Frazer, Dr Hamid Ghoddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry J. McArdle, Mrs Rebecca McKenzie, Professor Clare Mills, Dr Antonio Peña-Fernández, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, and Professor Bruce Whitelaw.
5. References
Andersen, J.M., Barrangou, R., Hachem, M.A., Lahtinen, S.J., Goh, Y.J., Svensson, B., and Klaenhammer, T.R. (2012). Transcriptional analysis of prebiotic uptake and catabolism by Lactobacillus acidophilus NCFM. PloS one 7 (9) e44409. https://doi.org/10.1371/journal.pone.0044409
Andersen, J.M., Barrangou, R., Abou Hachem, M., Lahtinen, S.J., Goh, Y.J., Svensson, B., and Klaenhammer, T.R. (2013). Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC genomics 14 312. https://doi.org/10.1186/1471-2164-14-312
Benes, I., and Kotyk, A. (1976). Uptake and binding of dissaccharides in human erythrocytes. Canadian journal of biochemistry 54 (1) 99-101 https://doi.org/10.1139/o76-016
BfR and Bundesinstitut für Risikobewertung (2020). Allulose, sugar substitute: More data is required for a health assessment as a food ingredient. BfR opinion No. 001/2020 of 8 January 2020.
Brucher, B., and Häßler, T. (2019). Enzymatic Process for the Synthesis of Cellobiose. In Industrial Enzyme Applications, A.V.a.O. May, ed., pp. 167-178.
https://doi.org/10.1002/9783527813780.ch2_4
Cantarel, B.L., Lombard, V., and Henrissat, B. (2012). Complex carbohydrate utilization by the healthy human microbiome. PloS one 7 (6) e28742-e28742.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3374616/
Cobden, I., Dickinson, R.J., Rothwell, J., and Axon, A.T. (1978). Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. British medical journal 2 (6144) 1060.
https://doi.org/10.1136/bmj.2.6144.1060
Cobden, I., Rothwell, J., and Axon, A.T. (1980). Intestinal permeability and screening tests for coeliac disease. Gut 21 (6) 512-518.
https://doi.org/10.1136/gut.21.6.512
Cobden, I., Hamilton, I., Rothwell, J., and Axon, A.T. (1985). Cellobiose/mannitol test: physiological properties of probe molecules and influence of extraneous factors. Clinica chimica acta; international journal of clinical chemistry 148 (1) 53-62.
https://doi.org/10.1016/0009-8981(85)90300-6
Dahlqvist, A. (1962). Specificity of the human intestinal disaccharidases and implications for hereditary disaccharide intolerance. The Journal of Clinical Investigation 41 (3) 463-470. https://doi.org/10.1172/JCI104499
De la Fuente, E., Sanz, M.L., Martínez-Castro, I., and Sanz, J. (2006). Development of a robust method for the quantitative determination of disaccharides in honey by gas chromatography. Journal of Chromatography A 1135 (2) 212-218.
http://www.sciencedirect.com/science/article/pii/S0021967306017894
EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) (2010). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J 8 (3) 1462.
https://doi.org/10.2903/j.efsa.2010.1462
EFSA NDA Panel (EFSA Panel on Nutrition and Novel Foods), 2016. Guidance on the Preparation and Presentation of an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA Journal 2016, 14(11): 4594. https://doi.org/10.2903/j.efsa.2016.4594.
Gentinetta, E., Zambello, M., and Salamini, F. (1979). Free sugars in developing maize grain. Cereal Chemistry (USA).
Gill, S.R., Pop, M., Deboy, R.T., Eckburg, P.B., Turnbaugh, P.J., Samuel, B.S., Gordon, J.I., Relman, D.A., Fraser-Liggett, C.M., and Nelson, K.E. (2006). Metagenomic analysis of the human distal gut microbiome. Science 312 (5778) 1355-1359.https://pubmed.ncbi.nlm.nih.gov/16741115;
Gray G.M., and Santiago, N.A. (1969). Intestinal β-galactosidases: I. Separation and characterization of three enzymes in normal human intestine. The Journal of Clinical Investigation 48 (4) 716-728. https://doi.org/10.1172/JCI106029
Hamilton, I., Cobden, I., Rothwell, J., and Axon, A.T. (1982). Intestinal permeability in coeliac disease: the response to gluten withdrawal and single-dose gluten challenge. Gut 23 (3) 202210. https://doi.org/10.1136/gut.23.3.202
Ilhan, Z.E., Marcus, A.K., Kang, D.-W., Rittmann, B.E., and Krajmalnik-Brown, R. (2017). pH Mediated Microbial and Metabolic Interactions in Fecal Enrichment Cultures. mSphere 2 (3) e00047-00017. https://msphere.asm.org/content/msph/2/3/e00047-17.full.pdf
Lau, H.K. (1987). Physicochemical characterization of human intestinal lactase. Biochem J 241 (2) 567-572. https://pubmed.ncbi.nlm.nih.gov/3109378; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1147598/
Magnúsdóttir, S., Heinken, A., Kutt, L., Ravcheev, D.A., Bauer, E., Noronha, A., Greenhalgh, K., Jäger, C., Baginska, J., Wilmes, P., et al. (2017). Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nature Biotechnology 35 (1) 81-89. https://doi.org/10.1038/nbt.3703
Messinger, H., Winkler, A., and Bar, A. (2020). Genotoxic potential of Cellobiose. Regulatory toxicology and pharmacology: RTP 111 104554.
https://doi.org/10.1016/j.yrtph.2019.104554
Mika, A., Stepnowski, P., Kaska, L., Proczko, M., Wisniewski, P., Sledzinski, M., and Sledzinski, T. (2016). A comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight. Obesity 24 (8) 1669-1676.
https://onlinelibrary.wiley.com/doi/abs/10.1002/oby.21560
Moinuddin, J.F., and Lee, H.W.-T. (1958). Effects of Feeding Diets Containing Sucrose, Cellobiose or Glucose on the Dry Weights of Cleaned Gastrointestinal Organs in the Rat. American Journal of Physiology-Legacy Content 192 (2) 417-420.
https://journals.physiology.org/doi/abs/10.1152/ajplegacy.1958.192.2.417
Moré, M.I., Postrach, E., Bothe, G., Heinritz, S., and Uebelhack, R. (2019). A Dose-
Escalation Study Demonstrates the Safety and Tolerability of Cellobiose in Healthy Subjects. Nutrients 12 (1) 64. https://www.mdpi.com/2072-6643/12/1/64
Morita, T., Ozawa, M., Ito, H., Kimio, S., and Kiriyama, S. (2008). Cellobiose is extensively digested in the small intestine by β-galactosidase in rats. Nutrition 24 (11) 1199-1204. http://www.sciencedirect.com/science/article/pii/S089990070800302X
Muflih, I.W., and Widdas, W.F. (1976). Sugars and sugar derivatives which inhibit the shortcircuit current of the everted small intestine of the rat. The Journal of physiology 263 (2) 101114.
https://doi.org/10.1113/jphysiol.1976.sp011623
Nakamura, S., Oku, T., and Ichinose, M. (2004). Bioavailability of Cellobiose by tolerance test and breath hydrogen excretion in humans. Nutrition 20 (11) 979-983. http://www.sciencedirect.com/science/article/pii/S0899900704001881
OECD, 1997. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https://doi.org/10.1787/9789264078536-en
OECD, 1998. OECD Principles on Good Laboratory Practice, OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, No. 1, OECD Publishing, Paris.
OECD, 2014. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Publishing, Paris. https://doi.org/10.1787/9789264224438-en
Potier, M., Dallaire, L., and Melancon, S.B. (1975). Occurrence and properties of fetal intestinal glycosidases (disaccharidases) in human amniotic fluid. Biology of the neonate 27 (3-4) 141-152.
https://doi.org/10.1159/000240771
Roberts, K.R., and Hayes, M.L. (1980). Effects of 2-deoxy D-glucose and other sugar analogues on acid production from sugars by human dental plaque bacteria. Scandinavian journal of dental research 88 (3) 201-209.
https://doi.org/10.1111/j.1600-0722.1980.tb01215.x
Sanz, M.L., Sanz, J., and Martínez-Castro, I. (2004). Gas chromatographic–mass spectrometric method for the qualitative and quantitative determination of disaccharides and trisaccharides in honey. Journal of Chromatography A 1059 (1) 143-148. http://www.sciencedirect.com/science/article/pii/S0021967304018114
Skovbjerg, H., Sjöström, H., and Norén, O. (1981). Purification and Characterisation of Amphiphilic Lactase/Phlorizin Hydrolase from Human Small Intestine. European Journal of Biochemistry 114 (3) 653-661. https://febs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1432https://febs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1432-1033.1981.tb05193.x1033.1981.tb05193.x
Strobel, S., Brydon, W.G., and Ferguson, A. (1984). Cellobiose/mannitol sugar permeability test complements biopsy histopathology in clinical investigation of the jejunum. Gut 25 (11) 1241-1246. https://gut.bmj.com/content/gutjnl/25/11/1241.full.pdf
Unpublished report, 2013. Resistance to intestinal brush border enzymes and to intestinal absorption, Adisseo.
Unpublished report, 2017a. Mutagenicity study of cellobiose in the Salmonella typhimurium and Escherichia coli reverse mutation assay (in vitro) according to Regulation (EC) No. 440/2008 method B.13/14 and OECD Guideline 471, Laboratory of Pharmacology and Toxicology GmbH. Study no. 32943.
Unpublished report, 2017b. In vitro assessment of cellobiose in the micronucleus test in cultured human peripheral lymphocytes according to OECD guideline 487, Laboratory of Pharmacology and Toxicology GmbH. Study no.32944.
Unpublished report, 2017c. 28-day dose-range-finding study of cellobiose by repeated oral administration via the drinking water in rats, Laboratory of Pharmacology and Toxicology GmbH. Study no. 32941.
Unpublished report, 2017d. 90-day repeated dose toxicity study of cellobiose by oral administration via the drinking water in rats, Laboratory of Pharmacology and Toxicology GmbH. Study no. 32942.
Unpublished report, 2021. A randomised, crossover, simple blind, referent-controlled study to determine Glycemic Index and Insulinemic Index of D-cellobiose and D-allulose, Biofortis.
Winkler, A., Messinger, H., and Bar, A. (2020). Subchronic (91-day) oral toxicity study of Cellobiose in rats. Regulatory toxicology and pharmacology: RTP 110 104518.
https://doi.org/10.1016/j.yrtph.2019.104518
Wu, M.-C., Chen, Y.-C., Lin, T.-L., Hsieh, P.-F., and Wang, J.-T. (2012). Cellobiose-Specific Phosphotransferase System of Klebsiella pneumonia and Its Importance in Biofilm Formation and Virulence. Infection and Immunity 80 (7) 2464-2472.
https://iai.asm.org/content/iai/80/7/2464.full.pdf
Yan, Y., Wang, Z., Greenwald, J., Kothapalli, K.S.D., Park, H.G., Liu, R., Mendralla, E., Lawrence, P., Wang, X., and Brenna, J.T. (2017). BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins, Leukotrienes and Essential Fatty Acids 116 27-31. https://doi.org/10.1016/j.plefa.2016.12.001
van Zanten GC, Krych L, Roytio H, Forssten S, Lahtinen SJ, Abu Al-Soud W, Sorensen S, Svensson B, Jespersen L andJakobsen M, 2014. Synbiotic Lactobacillus acidophilus NCFM and cellobiose does not affect human gut bacterialdiversity but increases abundance of lactobacilli, bifidobacteria and branched-chain fatty acids: a randomized,double-blinded cross-over trial. FEMS Microbiol Ecol, 90, 225–236. https://doi.org/10.1111/1574-6941.12397
Abbreviations
|
ACNFP |
Advisory Committee on Novel Foods and Processes |
|
ADME |
Absorption, Distribution, Metabolism and Excretion |
|
AE |
Adverse Events |
|
AOAC |
Association of Official Analytical Chemists |
|
BCFA |
Branched Chain Fatty Acids |
|
Bid |
Bis in Die (Twice Daily) |
|
BSA |
Bovine Serum Albumin |
|
BSFS |
Bristol Stool Form Scale |
|
Bw |
Body Weight |
|
Ds |
Dry Solids/Substance |
|
Dietex |
Dietary Exposure |
|
EDI |
Estimated Daily Intakes |
|
EFSA |
European Food Safety Authority |
|
FAIM |
Food Additives Intake Model |
|
FSA |
Food Standards Agency |
|
FSS |
Food Standards Scotland |
|
G1P |
Glucose-1-Phosphate |
|
GSRS |
Gastrointestinal Symptom Rating Scale |
|
HACCP |
Hazard Analysis and Critical Control Points |
|
LC-MS/MS |
Liquid Chromatography Tandem Mass Spectrometry |
|
LOQ |
Limit of Quantification |
|
LOD |
Limit of Detection |
|
MAD |
Multiple Ascending Dose |
|
Mos |
Margin of Safety |
|
NDA |
Dietetic Products, Nutrition and Allergies Panel |
|
NF |
Novel Food |
|
NMR |
Nuclear Magnetic Resonance |
|
NOAEL |
No Observed Adverse Event Level |
|
OECD |
Organisation for Economic Co-Operation And Development |
|
PTS |
Phosphotransferase System |
|
RCF |
Relative Centrifugal Force |
|
RDI |
Recommended Daily Intake |
|
RH |
Relative Humidity |
|
SAD |
Single Ascending Dose |
|
SCFA |
Short Chain Fatty Acids |
|
USP |
United States Pharmacopoeia |