ACNFP advice on the safety of 6’-sialyllactose (6’-SL) sodium salt as a novel food for use in food and food supplements
On this page
Skip the menu of subheadings on this page.Reference Number RP1478
Regulated Product Dossier Assessment
Assessment finalised: 10th of June 2024
Summary
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in March 2022, from Kyowa Hakko Bio Company Ltd, Japan (“the applicant”), for the authorisation of 6’-sialyllactose (6’-SL) sodium salt, as a novel food (NF).
The novel food is intended to be used as a source of a human identical milk oligosaccharide, 6’-SL.
6’-SL sodium salt is manufactured by microbial fermentation using a genetically modified strain of Escherichia coli W, and then refined to yield the purified novel food.
This new application is seeking to use the novel food within the food categories: dairy products and analogues, bakery wares, foods for special groups, beverages, and food supplements. Food supplements are not intended to be used if other foods with added 6’-SL sodium salt or breast milk are consumed the same day.
To support the FSA and FSS in their evaluation of the application, the Advisory Committee on Novel Foods and Processes (ACNFP) were asked to review the safety dossier and supplementary information provided by the applicant. The Committee did not consider any potential health benefits or claims arising from the consumption of the novel food, as the focus of the assessment is to ensure the food is safe and is not nutritionally disadvantageous for consumers.
The Committee concluded that the applicant had provided sufficient information to assure the novel food was safe under the conditions of use. The anticipated intake level and the proposed use in food and food supplements was not considered to be nutritionally disadvantageous.
1. Introduction
1. The ACNFP assessed the food safety risks of the novel food and its production under the proposed uses, in line with Article 7 of assimilated Commission Implementing Regulation (EU) 2017/2469. The regulatory framework and the retained technical guidance published by EFSA for full novel food applications is applicable to extension of use applications and formed the basis and structure for the assessment (EFSA NDA Panel, 2021).
2. In March 2022, Kyowa Hakko Bio Company Ltd, Japan (“the applicant”) submitted a full novel food application for the authorisation of 6’-sialyllactose sodium salt (6’-SL). The novel food is a water soluble white to off-white powder which is manufactured by microbial fermentation using a genetically modified strain of Escherichia coli W. 6’-SL sodium salt is intended to be used as a source of human identical milk oligosaccharide.
3. Following the review by the ACNFP in September 2023, further information was requested from the applicant concerning the identity, the production process, the compositional information, the stability, the nutritional information, and allergenicity information on 6’-SL sodium salt, in order to address information gaps in the initial dossier. The final advice from the Committee was agreed at the 165th meeting, allowing the FSA and FSS to complete the risk assessment.
4. This Committee advice document (CAD) outlines the conclusions of the ACNFP on the safety of the novel food.
2. Assessment
2.1 Identity of the novel food
5. The novel food is a white to off-white powder which is mainly composed of 6’-SL sodium salt (≥ 82.0% w/w DM). Other saccharides are present in smaller quantities: sialic acid (≤ 6.0% w/w DM), D-glucose (≤ 3.0% w/w DM), D-lactose (≤ 3.0% w/w DM), 6’-sialyllactulose and 3’-SL sodium salt (≤ 5.0% w/w DM, sum of both) and a small fraction of other related saccharides (sum of other carbohydrates ≤ 13.0% w/w DM).
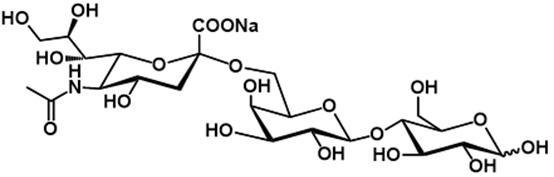
6. 6’-SL is a trisaccharide consisting of D-glucose, D-galactose, and sialic acid (N-acetyl neuraminic acid) (see figure 1). This is identical to the structure of 6’-SL in human breast milk.
Figure 1: The structural formula of 6’-SL
7. 6’-SL is classified as a purified chemical substance and characterised by the following information:
IUPAC name Sodium; (2R,4S,5R,6R)-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]-2-[[(2R,3R,4S,5R,6S)-3,4,5-trihydroxy-6-[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-
3-yl] oxyoxan-2-yl] methoxy]oxane-2-carboxylate
CAS number 157574-76-0
Molecular weight 655.53 g/mol
Molecular formula C23H38NO19Na
8. The structure of 6’-SL sodium salt in the novel food was confirmed using liquid chromatography – tandem mass spectrometry (LC–MS/MS) and nuclear magnetic resonance (NMR) spectroscopy: 1H-NMR, 13C-NMR, and two-dimensional (2D) NMR studies including COSY (correlation spectroscopy), TOCSY (total correlation spectroscopy), HETCOR (heteronuclear correlation) and HMBC (heteronuclear multiple bond correlation) methodologies.
9. 2D-NMR analysis using high purity standards of 6’-SL sodium salt and 3’-SL sodium salt, a conformational isomer of 6’-SL sodium salt, demonstrated that the sialic acid is linked to D-galactose by α-(2’’-6’) bonds. This provides unequivocal evidence on the structure of 6’-SL sodium salt.
10. High-performance liquid chromatography – charged aerosol detection (HPLC-CAD) was used to characterise 6’-SL sodium salt in eight batches of the novel food – five batches manufactured using soy peptone in the fermentation media and three batches manufactured without soy peptone in the fermentation media.
2.2 Production Process
11. The production microorganism, E. coli NE06, used to manufacture the novel food is a genetically modified derivative of E. coli W (Waksman’s strain) that functions as a processing aid as defined in Article 3(2)(b) of assimilated Regulation (EC) No.1333/2008 on food additives. A novel food produced by a GMO does not fall under the remit of the GMO legislation, assimilated Regulation (EC) No 1829/2003 or assimilated Regulation (EC) No 1830/2003, when the production microorganism is removed during the manufacturing process and therefore no recombinant DNA remains. This has been confirmed in the compositional analysis as detailed below.
12. The novel food is classified as category 1 under the EFSA GMO guidance: chemically defined purified compounds and their mixtures in which both genetically modified microorganisms (GMMs) and newly introduced genes have been removed, under EFSA guidance, which categorises GMMs and their products for risk assessment purposes (EFSA GMO Panel, 2011), which the FSA have retained for the purposes of technical review.
13. Although E. coli is not considered to be suitable for qualified presumption of safety (QPS) status (EFSA BIOHAZ Panel, 2023), E. coli W is widely used for biotechnological applications. Genomic analysis confirms that the genes required for pathogenicity are missing key components or they have been mutationally inactivated (Archer et al., 2011). Furthermore, E. coli W is considered to be a safe and non-pathogenic microorganism because this does not cause disease in healthy adult humans or colonise the human gut (Bauer et al., 2008; NIH, 2019). On the basis of this information, the new production strain organism does not introduce any new risks that need to be evaluated and managed.
14. The absence of bacteria from the Enterobacteriaceae family (ISO 21528-1:2017) and residual bacterial DNA (LOQ = 4 µg/kg) confirms the genetically modified E. coli NE06 is not present in the novel food.
15. The first stage of the production process involves the conversion of D-lactose and D-glucose to 6’-SL by the adapted cellular metabolism of the production microorganism (The commercial production process does not use soy peptone). Glucose acts as an exclusive energy and carbon source, and lactose as a substrate for the biosynthesis of 6’-SL. The 6’-SL is released from the E. coli NE06 into the fermentation broth. The production microorganism, E. coli NE06, is removed from the culture medium by microfiltration at the end of the fermentation process.
16. The second stage of the production process involves a series of purification and isolation steps (filtration, ion exchange, concentration and spray drying) to the obtain the final purified novel food in powder form.
17. Information on the acceptance criteria for the raw materials and processing aids was provided. The purity criteria for the reagents used in the manufacture of 6’-SL sodium salt are listed in Table 1.
Table 1: Purity criteria for reagents used to synthesize the novel food
|
Raw material |
Purity |
|
Glucose (powdered) |
≥ 89% (dextrose equivalent) |
|
Glucose (liquid) |
≥ 96% (dextrose equivalent) |
|
D-Lactose |
Optical rotation [α] 20D +54.4 to +55.9o |
18. The novel food is produced in line with Hazard Analysis and Critical Control Point (HACCP) principles. The manufacturing facility is FSSC (Food Safety System Certification) 22000 certified.
19. Bacterial contamination of the novel food is controlled by monitoring the purification steps and the membrane filtration step prior to spray drying. To prevent bacterial growth, the water content in the finished product is monitored and specified at ≤ 10.5% w/w. Endotoxin levels in the novel food are controlled by ultrafiltration and compliance with the specified level at ≤ 10 EU/mg.
20. The production process has characterised the potential hazards and the corresponding control measures are appropriate.
2.3 Compositional information
21. Results from five independent batches of 6’-SL sodium salt manufactured using fermentation media containing soy peptone and three independent batches of 6’-SL sodium salt manufactured using fermentation media without soy peptone demonstrated that the novel food is appropriately characterised (Table 2).
Table 2. Compositional Analysis of the novel food produced with and without soy peptone in fermentation media
|
Test Parameter |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
Batch 6 |
Batch 7 |
Batch 8 |
|
6’-SL sodium salt (% w/w DM) |
87 |
92 |
90 |
92 |
92 |
91 |
93 |
95 |
|
Sialic acid (% w/w DM) |
5.1 |
3.5 |
4.9 |
4.3 |
5.4 |
1.8 |
0.1 |
0.2 |
|
D-glucose (% w/w DM) |
ND |
ND |
ND |
ND |
ND |
< 0.05 |
< 0.05 |
< 0.05 |
|
D-lactose (% w/w DM) |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
< 0.05 |
< 0.05 |
< 0.05 |
|
6’-sialyllactulose (% w/w DM) |
0.4 |
0.4 |
0.5 |
0.5 |
0.4 |
----- |
----- |
----- |
|
3’-SL sodium salt (% w/w DM) |
ND |
ND |
ND |
ND |
ND |
----- |
----- |
----- |
|
6’-sialyllactulose and 3’-SL sodium salt (% w/w DM) |
---- |
----- |
----- |
----- |
----- |
1.7 |
0.5 |
0.3 |
|
Sum of other carbohydrates (% w/w DM) |
6.6 |
3.3 |
3.8 |
2.5 |
1.4 |
5.0 |
5.8 |
3.8 |
|
Water (%) |
5.3 |
5.0 |
5.4 |
5.6 |
5.0 |
7.9 |
8.4 |
8.6 |
|
Residual Protein (mg/kg) |
----- |
----- |
≤ 100 |
----- |
----- |
≤ 100 |
≤ 100 |
≤ 100 |
|
Sodium (% w/w DM) |
3.8 |
3.8 |
3.8 |
3.7 |
3.8 |
3.5 |
3.5 |
3.6 |
|
pH (5% solution, 25oC) |
6.4 |
6.5 |
6.5 |
6.5 |
6.5 |
6.1 |
5.9 |
5.8 |
|
Arsenic (mg/kg) |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
< 0.01 |
< 0.01 |
< 0.03 |
|
Cadmium (mg/kg) |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
< 0.01 |
< 0.01 |
< 0.01 |
|
Lead (mg/kg) |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
< 0.02 |
0.03 |
< 0.02 |
|
Mercury (mg/kg) |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
≤ 0.05 |
< 0.004 |
< 0.004 |
< 0.004 |
|
Aflatoxin M1 (µg/kg) |
----- |
< 0.02 |
< 0.02 |
----- |
----- |
< 0.02 |
< 0.02 |
< 0.02 |
|
Total plate count (CFU/g) |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
|
Yeast and mould (CFU/g) |
< 100 |
< 100 |
< 100 |
< 100 |
< 100 |
< 10 |
< 10 |
< 10 |
|
Enterobacteriaceae (in 10g) |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Salmonella (in 25g) |
----- |
----- |
ND |
----- |
----- |
ND |
ND |
ND |
|
Cronobacter spp. (in 10g) |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Listeria monocytogenes (in 25g) |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Presumptive Bacillus cereus (CFU/g) |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
|
Residual Endotoxins (EU/mg) |
0.006 |
0.011 |
0.020 |
0.034 |
0.026 |
< 0.0002 |
< 0.0002 |
< 0.0002 |
Batches 1 – 5 manufactured with soy peptone in fermentation media; batches 6 – 8 manufactured without soy peptone in fermentation media. SL = sialyllactose; DM = dry matter; ND = not detected; ---- = not determined; CFU = colony forming units; EU/mg = endotoxin units/mg.
For batches 1 -5, limit of detection (LOD) and limit of quantification (LOQ) respectively: sialic acid, 6’-sialyllactulose and 3’-SL sodium salts are 0.03% w/w DM and 0.2% w/w DM (as 6’-SL sodium salt); D-glucose and D-lactose is 0.02% w/w DM, and 0.05% w/w DM (as D-lactose). For batches 6 – 8, LOD and LOQ respectively: sialic acid = 0.15% w/w DM and 0.45% w/w DM (as sialic acid); 6’-sialyllactulose + 3’-SL sodium salts are = 0.13% w/w DM and 0.18% w/w DM (as 6’-SL sodium salt); D-glucose and D-lactose is 0.05% w/w DM.
Sum of other carbohydrates (w/w DM) = 100 – [6’-SL (acid) + quantified carbohydrates (i.e., sialic acid, D-glucose, D-lactose, 6’-sialyllactulose (acid) and 3’-SL (acid) + sodium].
LOQ for sodium = 1.25% (ICP-MS) for batches 1 – 5 and LOQ for sodium = 0.005% (ICP-OES) for batches 6 – 8; Protein test limit = 100 mg/kg; LOQ for heavy metals = 0.05 mg/kg for batches 1 – 5 and LOD and LOQ for As and Cd = 0.01 mg/kg and 0.03 mg/kg; LOD and LOQ for Pb = 0.02 mg/kg and 0.03 mg/kg; LOQ for Hg = 0.004 mg/kg and 0.01 mg/kg for batches 6 – 8; LOD for Aerobic plate count = 10 CFU/g; LOD for yeast and mould = 100 CFU/g (surface plating) for batches 1 – 5 and LOD for yeast and mould = < 10 CFU/g (in-depth plating) for batches 6 -8; LOD for Presumptive Bacillus cereus = 10 CFU/g; LOQ for endotoxins = 0.0001563 EU/mg (rounded up to 0.0002 EU/mg).
22. The composition data demonstrates that the novel food is consistently the same when the manufactured with or without soya peptone in the fermentation media.
23. Sialic acid, glucose, lactose, 6'-sialyllacultose, and 3'-SL sodium salt are naturally occurring components of human milk. Glucose is a breakdown product of the naturally occurring milk sugar lactose and 6'-sialyllactulose, is an isomerisation product of 6'-SL sodium salt, formed when the terminal glucose moiety isomerizes into fructose (EFSA NDA Panel, 2020).
24. An assessment was conducted in accordance with the EFSA Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles in the novel food (EFSA, 2021). A solubility in water test was conducted in two batches of the novel food produced with soy peptone and three batches without soy peptone in the fermentation media. The results confirmed that the novel food exceeded the decision criteria for solubility (> 33.3 g/L) as described in the guidance. Therefore, further evaluation for the presence of nanoparticles in the novel food is not required.
25. Certification was provided to demonstrate that the contract laboratories were accredited to perform these analytical studies. Where in-house analysis was utilised, full methodology and supporting validation documentation was provided.
26. The data presented indicate the novel food and any hazards present were appropriately characterised.
2.4 Stability
27. Results from an ongoing 36-month real-time stability study (25 ± 2°C; 60 ± 5% Relative Humidity) on a single batch of novel food produced with soy peptone in the fermentation media was provided. Data covering 24 months was reported. Testing for, carbohydrate, water content, physicochemical parameters, and water activity was undertaken at the 6- and 12-month time-points. These endpoints in addition to microbiology quality were assessed at 24 months. No significant changes were observed, and microbial parameters were below the limits of detection.
28. A 6-month accelerated stability study (40 ± 2°C; 75 ± 5% Relative Humidity) was conducted on five batches of novel food produced with soy peptone in the fermentation media. Data for the following parameters was provided: 6’-SL sodium salt, carbohydrate, sodium, water content, and physicochemical parameters and water activity. No significant changes were observed.
29. No stability data was provided for the novel food in different food matrices.
30. The presence of soy peptone in the fermentation media is not expected to have a significant impact on the composition of the novel food. On this basis, the data provided supports the stability of the novel food, manufactured with or without soy peptone in the fermentation media, for up to 24 months.
2.5 Specification
31. The specification parameters for the novel food (Table 3) were assessed using internationally recognised methods or are otherwise determined using internally developed and validated methods.
Table 3: Specification for the novel food
|
Parameter |
Specification |
Method |
|
6’-SL sodium salt |
≥ 82.0 % w/w DM |
HPLC-CAD (in-house) |
|
Sialic acid |
≤ 6.0 % w/w DM |
HPLC-CAD (in-house) |
|
D-glucose |
≤ 3.0 % w/w DM |
HPLC-PAD (in-house) |
|
D-lactose |
≤ 3.0 % w/w DM |
HPLC-PAD (in-house) |
|
Sum of 6’-sialyllactulose and 3’-SL sodium salt |
≤ 5.0 % w/w DM |
HPLC-CAD (in-house) |
|
Sum of other carbohydrates |
≤ 13.0 % w/w DM |
Calculation |
|
Water |
≤ 10.5 % w/w |
JP 2.48 |
|
Residual Protein |
< 0.01 % w/w |
Bradford assay |
|
Sodium |
≤ 5.0 % w/w DM |
USP <233> |
|
pH (5% solution, 25oC) |
4.5 – 7.5 |
JP 2.54 |
|
Arsenic |
≤ 0.2 mg/kg |
USP <233> or AOAC method |
|
Aflatoxin M1 |
≤ 0.025 µg/kg |
AOAC 2000.08 |
|
Total plate count |
≤ 1,000 CFU/g |
ISO 4833-1:2013 |
|
Yeast and mould |
≤ 100 CFU/g |
ISO 21527-2:2008 |
|
Enterobacteriaceae |
Absent in 10g |
ISO 21528-1:2017 |
|
Salmonella spp. |
Absent in 25g |
ISO 6579-1:2017 |
|
Cronobacter spp. |
Absent in 10g |
ISO 22964:2017 |
|
Listeria monocytogenes |
Absent in 25g |
ISO 11290-1:2017 |
|
Presumptive Bacillus cereus |
≤ 50 CFU/g |
ISO 7932:2004 |
|
Residual Endotoxins |
≤ 10 EU/mg |
JP 4.01 |
DM = dry matter; CFU = colony forming units; HPLC-CAD = high-performance liquid chromatography – charged aerosol detection; HPLC-PAD = high-performance liquid chromatography – pulsed amperometric detection; JP = Japanese Pharmacopoeia; USP = United States Pharmacopoeia; EPA = Environmental Protection Agency; ICP-MS = Inductively coupled plasma – mass spectrometry; ICP-OES = Inductively coupled plasma – optical emission spectroscopy; AOAC = Association of Official Analytical Chemists; ISO = International Organisation for Standardisation.
6’-sialyllactulose and 3’-SL sodium salts peaks on the HPLC-CAD chromatograms overlap.
Sum of other carbohydrates (w/w DM) = 100 – [6’-SL (acid) + quantified carbohydrates (i.e., sialic acid, D-glucose, D-lactose, 6’-sialyllactulose (acid) and 3’-SL (acid) + sodium].
Hg – EPA (2007) 7473.
32. The information provided is sufficient for the specification of the novel food, and appropriately characterises the novel food seeking authorisation.
2.6 History of Use
33. There is no history of use for the novel food in the UK.
34. 6’-SL sodium salt, which is a major constituent of the novel food, has been authorised in EU (assimilated Commission Implementing Regulation (EU) 2021/82 and assimilated Commission Implementing Regulation (EU) 2023/948) for use in a range of foods and food supplements. The authorised 6’-SL sodium salt is produced by fermentation using a genetically modified strains of E. coli K-12 DH1 and E. coli BL21 (DE3), respectively.
35. 6’-SL has been detected in bovine milk ranging from range from 4 to 10 mg/L and up to 100 mg/L in bovine colostrum (Aldredge et al., 2013; Urashima et al., 2013; Albrecht et al., 2014).
36. Human breast milk contains a family of structurally related oligosaccharides, known as human milk oligosaccharides (HMOs), as the third largest solid components (Kunz and Rudloff, 1993; Bode, 2012; Newburg, 2013). The concentrations of HMOs in human colostrum are 20 to 25 g/L, whereas in mature human milk, the concentrations are 5 to 20 g/L (Thurl et al., 2010; Bode, 2012; Urashima et al., 2018).
37. 6’-SL belongs to the subfraction of ‘acidic’ HMOs, which is characterised by the presence of sialic acids, and the whole subfraction accounts for 1.5 – 3.3 g/L (Thurl et al., 2010; Rijnierse et al., 2011; Bode, 2012). 6’-SL is the predominant acidic HMO and one of the most abundant HMOs along with 2’-FL (2’-fucosyllactose), lacto-N-fucopentaose I, LNT (lacto-N-tetraose) and LNnT (lacto-N-neotetraose).
38. There are two naturally occurring sialyllactoses, which are constitutional isomers. 3’-SL is sialylated by α-(2-3) linkage and 6’-SL is sialylated by α-(2-6) linkage. 6’-SL is the predominant acidic HMO in human milk; however, both forms are reported to have similar functions and biological roles (Tarr et al., 2015).
39. Thurl et al. (2017) summarised the findings from twenty-one published studies and reported that the content of 6’-SL in milk from mothers who delivered at term ranged from 0.38 to 0.91 g/L (average 0.64 g/L). For mothers who delivered preterm, 6’-SL ranged from 0.25 to 1.08 g/L (average 0.66 g/L). More recently, Soyyılmaz et al. (2021) reported that the mean of mean concentrations for mature and transitional milk were 0.40 g/L and 0.71 g/L respectively. The maximum means were 0.74 and 1.30 g/L, respectively.
40. Using the reported levels of 6’-SL in human breast milk from Thurl et al. (2017) and considering the average and high daily intake of breast milk (800 mL and 1,200 mL, respectively) for infants from 0 to 6 months (EFSA NDA Panel, 2013), the daily intake levels of 6’-SL from human milk for a 6.7 kg body weight infant (EFSA SC, 2012) are shown in Table 4.
Table 4: Estimated Intakes of 6’-SL from 800ml and 1200ml of Breast milk for 6.7kg Infant a using data from Thurl et al. (2017)
|
|
Mean intake levels (mg/kg BW/day) |
95th percentile intake levels (mg/kg BW/day) |
|
Estimated highest intake for 800ml milk * |
79 |
129 |
|
Estimated highest intake for 1200ml milk * |
118 |
193 |
* EFSA NDA Panel, 2013
41. The same approach, but using data from Soyyılmaz et al. (2021), results in lower intakes for 6’-SL, up to 133 mg/kg BW/day (EFSA NDA Panel, 2022).
42. The history of use does not indicate any further areas for evaluation.
2.7 Proposed Use and Anticipated Intake
43. The target population is the general population.
44. The intended uses and use levels of the 6’-SL sodium salt from the novel food are listed in Table 5. These food categories and intended use levels are the same as those assessed by EFSA for 6’-SL produced by fermentation with a genetically modified strain of E. coli K-12 DH1 (EFSA NDA, 2020).
Table 5: Food Categories and Use Levels for 6’-SL from the novel food
|
Food Category Name |
Proposed Maximum Use Level |
|
Unflavoured pasteurised and unflavoured sterilised (including UHT) milk products |
0.5 g/L |
|
Unflavoured fermented milk-based products |
0.5 g/L beverages 2.5 g/kg for products other than beverages |
|
Flavoured fermented milk-based products including heat-treated products |
0.5 g/L beverages 5 g/kg for products other than beverages |
|
Fine bakery wares. Cereal bars only |
5.0 g/kg |
|
Infant formula as defined in assimilated Regulation (EU) No 609/2013 |
0.4 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
|
Follow-on formula as defined in assimilated Regulation (EU) No 609/2013 |
0.3 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
|
Processed cereal-based food and baby food for infants and young children as defined in assimilated Regulation (EU) No 609/2013 |
0.3 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer 2.5 g/kg for products other than beverages |
|
Milk-based drinks and similar products intended for young children |
0.3 g/L (beverages) in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer 2.5 g/kg for products other than beverages |
|
Foods for special medical purposes as defined in assimilated Regulation (EU) No 609/2013 |
In accordance with the particular nutritional requirements of the persons for whom the products are intended |
|
Total diet replacement for weight control as defined in assimilated Regulation (EU) No 609/2013 |
1.0 g/L beverages (equivalent to 0.25 g/meal based on a standard 250 g/meal replacement beverage) 10 g/kg for products other than beverages (equivalent to 0.30 g/meal based on a standard 30 g meal replacement bar) |
|
Flavoured drinks (excluding cola-flavour and cola-flavoured drinks) |
0.5 g/L |
|
Food supplements * |
1.0 g/day for the general population; 0.3 g/day for young children; 0.4 g/day for infants |
* Food Supplements as defined in the Food Supplements (England) Regulations 2003, the Food Supplements (Wales) Regulations 2003 and the Food Supplements (Scotland) Regulations 2003.
45. The anticipated intake for 6’-SL sodium salt in children up to the age of 16 weeks is estimated to be 104 mg/kg body weight/day for a 6.7 kg infant. This value was calculated from the use of 6’-SL in infant formula (0.4 g/L) at a high consumption level of 260 ml/kg body weight/day, as established by the EFSA Scientific Committee (EFSA SC, 2017). This value does not exceed the estimated high daily intake of 6’-SL in breast-fed infants per kg/BW (see Table 4).
46. An intake assessment using the summary statistics of consumption from the dietary surveys in the EFSA Comprehensive database was conducted by matching the intended conditions of use with the FoodEx2 categories. The estimated mean and high-level intakes of 6’-SL sodium salt from the proposed conditions of use for each sub-population are presented in Table 6.
47. The highest estimated 95th percentile intake for 6’-SL sodium salt from the novel food was reported in the infant sub-population at 192 mg/kg BW/day. By comparison, the highest estimated intake for 6’-SL from human breast milk consumption is 193 mg/kg BW/day in infants (Table 4).
Table 6: Estimated daily intake for 6’-SL sodium salt from the current authorised uses in the List of Novel Foods (assimilated Commission Implementing Regulation (EU) 2017/2470).
|
Population Group |
Mean Intakes of 6’-SL (mg/kg BW/day) |
95th percentile intakes of 6’-SL (mg/kg BW/day) |
|
Infants (≤ 11 months) |
16 – 74 |
38 – 192 |
|
Young children (12 to 35 months) |
15 – 46 |
39 – 147 |
|
Other children (3 to 9 years) |
6 – 25 |
16 – 61 |
|
Adolescents (10 to 17 years) |
3 – 8 |
7 – 19 |
|
Adults (18 to 64 years) |
1 – 5 |
5 – 13 |
|
Pregnant women |
1 – 5 |
4 – 11 |
|
Lactating women |
4 – 5 |
10 – 11 |
|
Elderly (> 65 years) |
1 – 4 |
5 – 10 |
48. The use level for 6’-SL sodium salt in food supplements is 0.4 g/day for infants, 0.3 g/day for young children, and 1 g/day for all other population sub-groups. Food supplements are not intended to be used if other foods with the novel food are consumed on the same day. For infants and young children, food supplements are not intended to be used if breast milk or other foods with added 6’-SL sodium salt are consumed on the same day.
49. On a body weight basis, the maximum intake of 6’-SL sodium salt in food supplements for infants (0.4 g/day) and young children (0.3 g/day) would be 60 mg/kg BW/day and 25 mg/kg BW/day, respectively. For all other sub-populations, the maximum daily intake would range from 14 to 43 mg/kg BW/day based on default body weight (EFSA SC, 2012). On this basis, consumption of 6’-SL sodium salt in food supplements is not expected to exceed the levels found in human breast milk.
50. Certain food categories for 6’-SL sodium salt are currently authorised (Assimilated Commission Implementing Regulation (EU) 2023/948) at higher use levels than those listed in Table 6. Therefore, an assessment of the combined intake of authorised and intended food categories was conducted. The estimated combined mean and 95th percentile intakes for each sub-population are presented in Table 7.
Table 7: Estimated daily intake for 6’-SL from the current authorised uses and intended uses
|
Population Group |
Mean Intakes of 6’-SL sodium salt (mg/kg BW/day) |
95th percentile intakes of 6’-SL sodium salt (mg/kg BW/day) |
|
Infants (≤ 11 months) |
23 – 95 |
66 – 206 |
|
Young children (12 to 35 months) |
11 – 47 |
29 – 159 |
|
Other children (3 to 9 years) |
4.1 – 15 |
9.5 – 26 |
|
Adolescents (10 to 17 years) |
0.7 – 5.8 |
3.0 – 13 |
|
Adults (18+ years) |
2.3 – 3.0 |
5.2 – 7.9 |
51. The highest combined estimated 95th percentile daily intake is reported in infants at 206 mg/kg BW/day. This is higher than the estimated highest concentration of 6’-SL sodium salt from the daily intake of human milk of 193 mg/kg BW/day (Table 4). The estimated 95th percentile intake levels for all other sub-population groups are not expected to exceed this value.
2.8 Absorption, Distribution, Metabolism and Excretion (ADME)
52. No ADME studies were conducted on the novel food.
53. 6’-SL does not undergo any significant digestion by human enzymes in the upper gastrointestinal tract and only small amounts are expected to be absorbed (EFSA NDA Panel, 2020). HMOs are fermented in the colon by intestinal microbiota with a fraction excreted unchanged in the faeces and a small fraction found in the urine (EFSA NDA Panel, 2022). There is no information which indicates that 6’-SL in the novel food differs from the 6’-SL in human breast milk (EFSA NDA Panel, 2023).
54. The ADME of human milk oligosaccharides are well understood and the information does not indicate any further areas of concern.
2.9 Nutritional information
55. The novel food is mainly composed of the oligosaccharide, 6’-SL as a sodium salt, which is structurally identical to the naturally occurring counterpart in human breast milk.
56. The novel food contains 6’-SL sodium salt which may contribute to the daily intake of sodium in consumers. The EFSA NDA Panel has provided advice on the levels of sodium that are considered adequate and safe (EFSA NDA Panel, 2019).
Table 8: Estimated highest daily intakes of sodium in all sub-populations from intended uses of 6’-SL sodium salt.
|
Population Group * |
Highest 95th percentile intakes of 6’-SL sodium salt ** (mg/day) |
Highest estimated intake of sodium from 6’-SL sodium salt (mg/day) |
EFSA adequate and safe intakes levels for sodium (mg/day) |
|
Children (≤ 16w) |
520 |
26 |
Not established |
|
Infants (≤ 11m) |
960 |
48 |
120 (≤ 6m) 200 (7 – 11m) |
|
Young children (12 – 35m) |
1,764 |
88 |
1100 |
|
Other children (3 – 9y) |
1,403 |
70 |
1300 (4 – 6y) 1700 (7 – 10y) |
|
Adolescents (10 – 17y) |
817 (10 – 14 y) 1,159 (15 – 17y) |
41 58 |
2000 (> 11y) |
|
Adults (> 18y) |
910 |
46 |
2000 |
w = weeks; m = months; y = years
* EFSA SC, 2012
** based on highest specified limit for sodium of 5% w/w in novel food (see Table 4)
57. The estimated highest intakes of sodium from 6’-SL consumption are shown in Table 8.
58. Consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
2.10 Toxicological information
59. Toxicological studies were performed with 6’-SL sodium salt to support the safety assessment of the novel food. The respective study reports are unpublished and claimed as proprietary data. They were reviewed by the ACNFP and considered essential in the assessment of the safety of the novel food.
2.10.1 Genotoxicity
60. In vitro genotoxicity testing of 6’-SL sodium salt was conducted under Good Laboratory Practice (GLP) conditions and according to the OECD guidelines: in vitro bacterial reverse mutation test (OECD TG 471) and in vivo mammalian erythrocyte micronucleus test (OECD TG 474). This is not the approach recommended by the UK Committee on Mutagenicity or in the guidance on the preparation and submission of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283.
61. The Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA, 2011) recommends two in vitro tests as the first step in testing for genotoxicity: in vitro bacterial reverse mutation test (OECD TG 471) and in vitro mammalian cell micronucleus test (OECD TG 487). This approach addresses the key endpoints for adequately assessing genotoxicity with the minimum number of tests and avoiding unnecessary animal tests.
62. The in vivo mammalian erythrocyte micronucleus test (OECD TG 474) assesses both structural and numerical chromosomal aberrations and is an appropriate follow-up test for in vitro clastogens and aneugens.
63. The in vitro bacterial reverse mutation test (Ogama, 2020 [unpublished]) demonstrated that 6’-SL sodium salt is non-mutagenic at concentrations up to 5,000 µg 6’-SL/plate, in the absence or presence of metabolic activation.
64. The in vivo mammalian micronucleus test (Kikuchi, 2020 [unpublished]) reported that that 6’-SL sodium salt is non-clastogenic and non-aneugenic. However, no evidence was provided to demonstrate that the bone marrow in mice had been exposed to 6’-SL. Therefore, an in vitro micronucleus test was conducted.
65. The in vitro micronucleus test (Kikuchi, 2022 [unpublished]) demonstrated that 6’-SL sodium salt is non-clastogenic and non-aneugenic in the absence or presence of metabolic activation up to the highest concentration of 2,000 µg 6’-SL/ml.
66. The results from these in vitro and in vivo studies support the conclusion that 6’-SL sodium salt is not genotoxic.
2.10.2 Sub-chronic toxicity
67. A Repeated Dose 90-day oral gavage study in rodents (Tsuboi, 2021 [unpublished]) was conducted under GLP conditions according to OECD TG 408 guidelines as recommended by the Guidance on the preparation and submission of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283. The aim of the study was to identify any adverse effects following the consumption of 6’-SL sodium salt.
68. In this 90-day oral gavage study, each group consisted of 10 female and 10 male Sprague Dawley rats which were dosed with 0 (control – vehicle only [water]), 542, 1,084 or 2,168 mg/kg BW/day of 6’-SL sodium salt by oral gavage.
69. No deaths, test item-related clinical abnormalities, ocular changes, or differences in food consumption and bodyweight between test groups were reported. Statistically significant changes in haematology, clinical chemistry, urinalysis, and organ weights were reported. However, these were not dose dependent and the applicant has confirmed these were within the historical control range for the facility and therefore not of concern.
70. No dose related abnormalities were noted during the necropsy or histopathological evaluation. Therefore, the no observable adverse effect level (NOAEL) for 6’-SL sodium salt was considered to be the highest dose tested of 2,168 mg/kg BW/day.
2.10.3 Human studies
71. No human clinical trials were conducted with the novel food.
2.11 Allergenicity
72. The protein content of the novel food is reported as < 0.01% w/w.
73. Absence of bacteria from the Enterobacteriaceae family (ISO 21528-1:2017) confirmed that the genetically modified E. coli NE06 is not present in the novel food.
74. The potential allergenicity of the introduced proteins expressed in E. coli W was assessed using the National Institute of Health Sciences (Japan) Allergen Database for Food Safety and conducted in line with FAO (2001) guidelines. None of the proteins was predicted to be an allergen.
75. ELISA (enzyme linked immunosorbent assay) tests were conducted on the novel food manufactured using soy peptone to detect the presence of milk proteins (LOQ = 1 µg/g). The results confirmed that milk proteins were effectively removed during the purification process and were not present in the finished powder.
76. Five batches of the novel food manufactured using soy peptone were also tested for the presence of soybean protein. The results from the ELISA test (LOQ = 1 µg/g) confirmed that soybean protein was effectively removed from all batches of the novel food during the purification process and was not present in the finished powder.
77. The likelihood of allergenic reactions to the novel food is expected to be low under the proposed conditions of use.
3. Discussion
78. The novel food is a white to off-white powder which is mainly composed of the human identical milk trisaccharide, 6’-SL as a sodium salt (≥ 82.0% w/w DM), as well as other saccharides in smaller quantities.
79. The novel food is manufactured by microbial fermentation using a genetically modified strain of Escherichia coli W and then refined to yield the purified powder.
80. 6’-SL sodium salt is intended to be used in dairy products and analogues, bakery wares, foods for special groups, beverages, and food supplements. The general population are identified as the target population of the novel food.
81. Analysis confirms that the 6’-SL sodium salt is structurally identical to the 6’-SL found naturally in human milk. Exposure to 6’-SL relates solely to breastfeeding infants as there is no recognised history of use for this milk oligosaccharide as an ingredient in foods or food supplements.
82. In the Repeated Dose 90-Day Oral Toxicity Study in Rodents, the NOAEL for 6’-SL sodium salt was 2,168 mg/kg BW/day, the highest dose tested. When this NOAEL is compared with the highest estimated exposure in each population category, the margins of exposure range from 11 to 217. Given that the 6’-SL sodium salt in the novel food is equivalent to 6’-SL found in human breast milk, these margins of exposure are acceptable with respect to the highest estimated daily intakes in the intended population.
83. For most sub-populations, the combined daily intake of the 6’-SL sodium salt is not expected to exceed the highest estimated intake level of 193 mg/kg BW/day for 6’-SL in breastfed infants. In the infant sub-population, the highest combined estimated intake of 6’-SL sodium salt is reported as 206 mg/kg BW/day, which exceeds this value. However, given the wide range of estimated intakes for 6’-SL sodium salt (66 – 206 mg/kg BW/day), the limited absorption of HMOs, and the absence of toxicological effects reported in the in vivo 90-day oral gavage study, this is not considered to be a concern.
84. The use level of 6’-SL sodium salt in food supplements (0.4 g/day for infants, 0.3 g/day for young children and 1.0 g/day for all other sub-populations) is not expected to exceed the highest intake level of 6’-SL in breastfed infants on a body weight basis. Food supplements are not intended to be used by the general population if other foods containing the novel food, including breast milk or other foods for infants and young children, are consumed on the same day.
4. Conclusion
85. The ACNFP have undertaken the assessment of the novel food, which is composed mainly of 6’-SL sodium salt, and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake level and the proposed use in food and food supplements was not considered to be nutritionally disadvantageous.
86. The advice was based on the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
- annexes to the dossier which relate to the identity of the novel food, the production process, composition, stability, and the solubility of the novel food.
- in vitro bacterial reverse mutation test (Ogama, 2020 [unpublished]); in vitro micronucleus test (Kikuchi, 2022 [unpublished]); 90-day repeat dose feeding study with the novel food (Tsuboi, 2021 [unpublished]).
The members of the ACNFP during the course of the assessment who were;
Dr Camilla Alexander White, Dr Anton Alldrick, Dr Kimon Andreas Karatzas, Ms Alison Austin, Professor George Bassel, Dr Mark Berry, Dr Christine Bosch, Professor Dimitris Charalampopoulos, Dr Catharina Edwards, Professor Susan Fairweather-Tait, Professor Paul Frazer, Dr Hamid Ghoddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry J. McArdle, Mrs Rebecca McKenzie, Professor Clare Mills, Dr Antonio Peña-Fernández, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, and Professor Bruce Whitelaw.
References
Albrecht S, Lane JA, Marino K, Al Busadah KA, Carrington SD, Hickey RM and Rudd PM, 2014. A comparative study of free oligosaccharides in the milk of domestic animals. British Journal of Nutrition, 111, 1313–1328.
https://doi.org/10.1017/s0007114513003772
Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB and Barile D, 2013. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology, 23, 664–676.
https://doi.org/10.1093/glycob/cwt007
Archer CT, Kim JF, Jeong H, Park JH, Vickers CE, Lee SY and Nielsen LK, 2011. The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome-scale reconstruction of E. coli. BMC Genomics, 12, 20.
https://doi.org/10.1186/1471-2164-12-9
Bauer AP, Ludwig W and Schleifer KH, 2008. A novel DNA microarray design for accurate and straightforward identification of Escherichia coli safety and laboratory strains. Systematic and Applied Microbiology, 31, 50–61.
https://doi.org/10.1016/j.syapm.2008.01.001
Bode L, 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22(9), 1147-1162. https://doi.org/10.1093/glycob/cws074
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2023. Scientific Opinion on the update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA Journal 2023;21(1):7747, 23 pp. https://doi.org/10.2903/j.efsa.2023.7747
FSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2011. Scientific Opinion on Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use (Question no EFSA-Q-2009-00521, adopted: 25 May 2009). EFSA Journal, 9(6):2193 [54 pp.].
https://doi.org/10.2903/j.efsa.2011.2193.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal, 11(10):3408 [103 pp.].
https://doi.org/10.2903/j.efsa.2013.3408.
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), 2019. Scientific opinion on the dietary reference values for sodium. EFSA Journal 2019; 17(9): 5778, [191 pp]. https://doi.org/10.2903/j.efsa.2019.5778
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), 2020. Scientific opinion on the safety of 6’-Sialyllactose (6’-SL) sodium salt as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2020;18(5):6097, 23 pp. https://doi.org/10.2903/j.efsa.2020.6097
EFSA NDA Panel (Panel on Dietetic Products, Nutrition and Allergies), 2021. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283 (Revision 1). Published 26 March. https://doi.org/10.2903/j.efsa.2011.2170
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), 2022. Scientific Opinion on the safety of 6’-sialyllactose (6’-SL) sodium salt produced by derivative strains of Escherichia coli BL21 (DE3) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2022;20(12):7645, [29 pp].
https://doi.org/10.2903/j.efsa.2022.7645
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), 2023. Opinion on the safety of 6’-sialyllactose (6’-SL) sodium salt produced by a derivative strain (Escherichia coli NEO6) of E. coli W (ATCC 9637) as a Novel Food pursuant to Regulation (EU) 2015/2283. EFSA Journal 21(6):8025, [23 pp].
https://doi.org/10.2903/j.efsa.2023.8025
EFSA, 2011.Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 9(9):2379 [69 pp.]
https://doi.org/10.2903/j.efsa.2011.2379
EFSA, 2012. Scientific Committee Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 10(3):2579. [32 pp.]
https://doi:10.2903/j.efsa.2012.2579.
EFSA Scientific Committee, 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (Question no EFSA-Q-2016-00489, adopted: 26 April 2017). EFSA Journal, 15(5):4849 [58 pp.].
https://doi.org/10.2903/j.efsa.2017.4849.
EFSA, 2021. Scientific Committee on Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 19(8):6769 [48 pp.]
https://doi.org/10.2903/j.efsa.2021.6769
FAO/WHO, 2001. Evaluation of allergenicity of genetically modified foods. Report of a Joint FAO/WHO Expert Consultation on Allergenicity of Food Derived from Biotechnology, 22-25 January 2001. Food and Agriculture organisation of the United Nations (FAO), Rome, Italy. https://cdn.who.int/media/docs/default-source/documents/publications/evaluation-of-allergenicity.pdf?sfvrsn=9528a2fb_2&download=true (Accessed March 2024).
Kikuchi M, 2020 [unpublished]. Dated 26 November 2020. Yoshimi Laboratories, Drug Safety Testing Center Co., Ltd, Saitama 355-0166, Japan. Study Title: Micronucleus Study of 6’-Sialyllactose sodium salt in Bone-marrow Cells of Mice (Study number: 200059)
Kikuchi M, 2022 [unpublished]. Dated 31 October 2022. Yoshimi Laboratories, Drug Safety Testing Center Co., Ltd, Saitama 355-0166, Japan. Study Title: Micronucleus Study of 6’-Sialyllactose sodium salt (6SL Na) in Cultured Mammalian Cells (Study number: CG220004)
Kim JH, Yong S-Y, Kim SH, Baek A, Go T-H and Kang D-R, 2022. Randomized, triple-blind, placebo-controlled study to evaluate the safety of 60-Sialyllactose in healthy adults. Regulatory Toxicology and Pharmacology, 129, 105–110.
https://doi.org/10.1016/j.yrtph.2021.105110
Kunz C and Rudloff S, 1993. Biological functions of oligosaccharides in human milk. Acta Paediatrica, 82(12), 903-912.
https://doi.org/10.1111/j.1651-2227.1993.tb12597.x
Newburg DS, 2013. Glycobiology of human milk. Biochemistry (Moscow), 78, 771-785 (Russian edition "Biokhimiya, 778, 990-1007").
https://doi.org/10.1134/s0006297913070092
NIH (National Institutes of Health), 2019. NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. National Institutes of Health (NIH), Office of Science Policy, Bethesda, MD Available online:
https://osp.od.nih.gov/biotechnology/nih-guidelines/ (accessed on 1 March 2024)
OECD, 1997. Bacterial reverse mutation test. In OECD guidelines for the testing of chemicals. OECD guideline No 471 (updated & adopted: 21 July 1997). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264071247-en
OECD, 1998. OECD principles of good laboratory practice. Series on principles of good laboratory practice and compliance monitoring, No. 1 (ENV/MC/CHEM(98) 17). Paris, France: Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Chemicals Group and Management Committee.
https://doi.org/10.1787/9789264078536-en
OECD, 2016a. Mammalian in vivo micronucleus test. In OECD guidelines for the testing of chemicals. OECD guideline No 474 (updated & adopted: 29 July 2016). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264264762-en
OECD, 2016b. In vitro mammalian cell micronucleus test. In OECD guidelines for the testing of chemicals. OECD guideline No 487 (updated & adopted: 29 July 2016). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264264861-en
OECD, 2018. Repeated dose 90-day oral toxicity study in rodents. In OECD guidelines for the testing of chemicals. OECD guideline No 408 (updated and adopted 27 June 2018). Paris, France: Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264070707-en
Ogama Y, 2020 [unpublished]. Dated 11 September 2020. Yoshimi Laboratories, Drug Safety Testing Center Co., Ltd, Saitama 355-0166, Japan. Study Title: A BACTERIAL REVERSE MUTATION TEST OF 6'-Sialyllactose sodium salt (Study number: AG200051).
Rijnierse A, Jeurink PV, van Esch BCAM, Garssen J and Knippels LMJ, 2011. Food-derived oligosaccharides exhibit pharmaceutical properties. European Journal of Pharmacology, 668, S117–S123. https://doi.org/10.1016/j.ejphar.2011.07.009
Soyyılmaz B, Miks MH, Reohrig CH, Matwiejuk M, Meszaros-Matwiejuk A and Vigsnæs LK, 2021. The mean of milk: a review of human milk oligosaccharide concentrations throughout lactation. Nutrients, 13, 2737.
https://doi.org/10.3390/nu13082737
Tarr AJ, Galley JD, Fisher S, Chichlowski M, Berg BM and Bailey MT, 2015. The prebiotics 3′-Sialyllactose and 6-Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain, Behavior, and Immunity, 50, 166–177. https://doi.org/10.1016/j.bbi.2015.06.025
Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J and Stahl B, 2010. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. British Journal of Nutrition, 104, 1261–1271. https://doi.org/10.1016/j.bbi.2015.06.025
Thurl S, Munzert M, Boehm G, Matthews C and Stahl B, 2017. Systematic review of the concentrations of oligosaccharides in human milk. Nutrition Reviews, 75(11), 920-933. https://doi.org/10.1093/nutrit/nux044
Tsuboi M, 2021 [unpublished]. Dated 26 February 2021. Yoshimi Laboratories, Drug Safety Testing Center Co., Ltd, Saitama 355-0166, Japan. Study Title: Ninety-Day Repeated Oral Dose Toxicity Study of 6'‐Sialyllactose sodium salt in Rats (Study number: 100603RG).
Urashima T, Taufik E, Fukuda K and Asakuma S, 2013. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Bioscience, Biotechnology, and Biochemistry, 77, 455–466. https://doi.org/10.1271/bbb.120810
Urashima T, Yamaguchi E, Ohshima T, Fukuda K and Saito T, 2018. Chemical structures of oligosaccharides in milk of the raccoon (Procyon lotor). Glycoconjugate Journal, 35, 275–286. https://doi.org/10.1007/s10719-018-9821-z