ACNFP Advice on the safety of Barley Rice Protein
On this page
Skip the menu of subheadings on this page.Reference Number RP19
Food Standards Agency (FSA) and Food Standards Scotland (FSS)
Regulated Product Dossier Assessment
Version 1
Assessment finalised: 14th of August 2023
Summary
An application was submitted to the Food Standards Agency in January 2021 from Evergrain, LLC, USA (“the applicant”) for the authorisation of Barley Rice Protein, a mixture of protein from barley at levels of 30-70% and rice at levels of 70-30%. The applicant intends to market the product within food categories including: bakery products, breakfast cereals, spreadable fats and dressings, grain products and pastas, snack foods, jam, marmalade and other fruit spreads, candy/confectionery, dairy and dairy imitates, dessert sauces and syrups, meat imitates, soups and soup mixes, savoury sauces, legume-based spreads, nut-based spreads, energy drinks, foods and beverages intended for sportspersons and meal replacements for weight control.
To support the Food Standards Agency (FSA) and Food Standard Scotland (FSS) in evaluating the dossier, the Advisory Committee on Novel Foods and Processes (ACNFP) was asked to review the dossier. The Committee concluded that Barley Rice Protein is safe under the proposed conditions of use, based on the composition and the anticipated intake. The Committee considered that the proposed uses were not nutritionally disadvantageous if used alone or in combination with other plant sources of protein. However, the Committee expressed concern that it may be nutritionally disadvantageous if used as a meat or dairy protein substitute in products marketed as meal replacements for weight control.
1. Introduction
- To support the risk assessment by FSA and FSS for Barley Rice Protein, ACNFP provided advice to the FSA and FSS outlined in this document.
- The Applicant is seeking to use the novel food ingredient Barley Rice Protein within the following food categories: bakery products, breakfast cereals, spreadable fats and dressings, grain products and pastas, snack foods, jam, marmalade and other fruit spreads, candy/confectionery, dairy and dairy imitates, dessert sauces and syrups, meat imitates, soups and soup mixes, savoury sauces, legume-based spreads, nut-based spreads, energy drinks, foods and beverages intended for sportsmen and meal replacements for weight control.
- A safety dossier submitted by the Applicant was evaluated by the ACNFP at their April 2021(footnote) meeting and again at their September 2021 (footnote) meeting. Requests for further information were sent to the applicant after each meeting. The applicant’s response to the request for further information from the September 2021 meeting was further evaluated at ACNFP’s February 2022 (footnote) meeting.
- This document outlines the conclusions of the Committee’s assessment on the safety of Barley Rice Protein which will inform the basis of the FSA and FSS view on the application for Barley Rice Protein, Reference RP19.
2. Assessment
2.2 Identity of the novel food
5. Barley Rice Protein was identified as a powdered mixture of protein from barley (Hordeum vulgare) at levels of 30 to 70% and rice (Oryza sativa) at levels of 70 to 30%. Barley leaf and grain/seed and rice seed would be the parts used to produce Barley Rice Protein, with ingredients originating from North America and Europe.
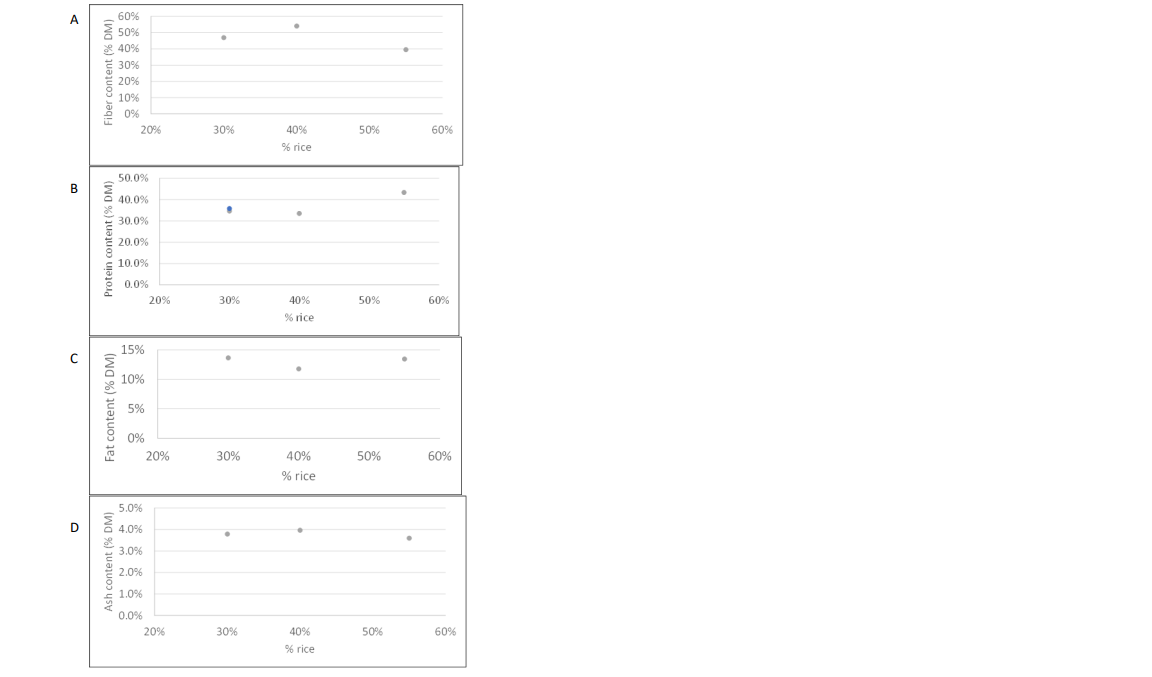
6. The ACNFP noted the raw materials used to produce Barley Rice Protein were a by-product from beer production and requested further information on the composition of the raw material. The applicant described how insoluble material containing the protein fraction, brewers spent grain (BSG), is isolated during the brewing process and is used as the raw material in the production of Barley Rice Protein. The applicant also provided Figure 1, which showed the generic composition of the material in the production of Barley Rice Protein.
Figure 1. Characterisation of A) Fibre B) Protein C) Fat and D) Ash Content in Brewers Spent Grain as a Function of Rice Content.
7. The ACNFP sought clarification from the applicant on whether the novel food is a protein mix or a protein hydrolysate. The applicant did not explicitly define the food as a hydrolysate and explained that glucoamylase is used to hydrolyse the starch and a protease to hydrolyse and solubilise the protein fraction, concluding that the product is “produced by selective isolation of the protein fraction of barley and rice”.
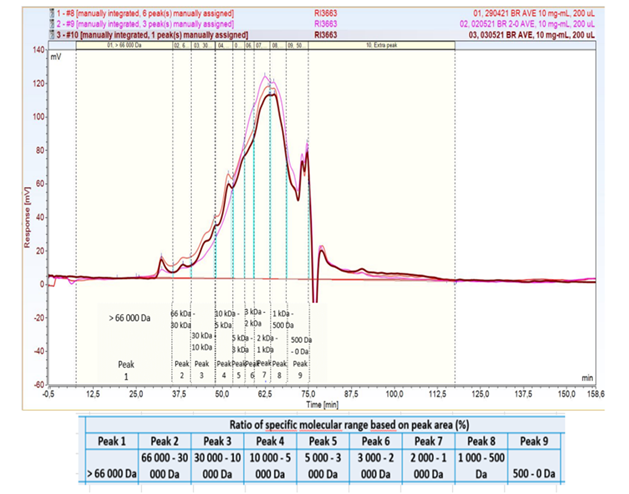
8. It was noted following the hydrolysis step a proportion of proteins were greater than 30kDa which would have implications for the allergenicity risk assessment. Further information was sought on particle size distribution of the product following hydrolysation and how a consistent product was achieved. The applicant provided details of the production process, stating high molecular weight fragments are removed (microfiltration; 0.1 to 0.5 µm cut-off), or low molecular weight proteins and peptides (nanofiltration; 500 to 1,500 Da cut-off) are removed. This allows for a consistently narrow molecular size distribution in the final product. Results provided from Figure 2 indicated the protein fraction of the Barley Rice Protein to be within the 500 Da to 3 kDa range. The evidence provided on protein size and that the product had similar properties to a protein hydrolysate was considered. Therefore, based upon the evidence, Barley Rice protein was treated as a protein hydrolysate in the relevant sections of the risk assessment in particular for the allergenicity review.
Figure 2. Molecular size distribution of 3 production batches of Barley Rice Protein.
NB. Figure 2 taken from the applicants data. Table data interpreted as Molecular Weight range, not ratio for ACNFP review.
9. The ACNFP sought further information on the variability of the level of addition for the two starting materials and the implications this would have on the composition of Barley Rice Protein. The applicant provided further information on the management of the starting material, by presenting details on the composition and proportions of starting material used. Additional information was also provided to illustrate consistency of the enzymatic hydrolysation step and the level of variability in the end product. As a result of these discussions the applicant amended their specification so that the ratio for barley is 70-30% and rice is 30-70%.
10. Compositional analysis of batches of the novel food demonstrated that despite the variability in the ratios of barley and rice in the starting material of brewers spent grain, a consistent product was yielded with no appreciable differences in the fibre, protein, fat, or ash content.
2.3 Production Process
11. Barley Rice Protein is manufactured using primarily mechanical processes, the chemicals used are pH adjusting agents; potassium hydroxide and sodium hydroxide. Food-grade glucoamylase and food-grade protease are also used. These enzymes comply with the specifications for enzyme preparations established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (JECFA, 2006) and Food Chemicals Codex (FCC) (FCC, 2018) and are used at levels in accordance with current Good Manufacturing Practice (cGMP). The enzymes are derived from non-toxigenic and non-pathogenic sources; therefore, they are not expected to be of safety concern when used in the production of Barley Rice Protein.
12. The barley and rice mixture from the mash step of beer production is treated with glucoamylase to hydrolyse the starch; the pH is then adjusted, and protease is added to hydrolyse the protein component. Enzymes are deactivated by heat treatment and the resulting mixture is then purified, filtered, and concentrated and spray dried to yield the final powdered Barley Rice Protein.
13. Based upon the advice of the ACNFP, further information was sought from the FSA on the steps of the production process to better understand whether the key hazards had been identified and controlled. The applicant responded by providing additional information on the process and the products in each fraction of the filtration process.
14. Based upon the advice of the ACNFP, further information was requested by the FSA on the consistency of the enzyme digestion element of the production process and the level of potential variability. The applicant responded that the method provided a consistent output, for this the molecular weight profiles of 3 production batches of Barley Rice Protein were analysed by high-performance size exclusion chromatography. The molecular size distribution of 3 production batches of Barley Rice Protein were overlayed to demonstrate the consistency across the production batches (Figure 2.)
15. The applicant was also asked to provide details of the conditions of the process which resulted in inactivation of enzymes in the final product and data were provided to demonstrate this. The additional information provided evidence of the control of the process. It was advised by the ACNFP that FSA risk managers may wish to consider inclusion of additional parameters to the specification to support this, ensuring consistency in the hydrolysation step.
2.4 Composition
16.The applicant provided data sets on the composition of Barley Rice Protein. Information on proximate analysis of 5 different batches was provided (Table 1).
Table 1. Results of proximate analysis of 5 non-consecutive batches of Barley Rice Protein
|
Parameter |
Specification Limit |
Manufacturing Lot Number: 080318SP01 |
122018BRSP01 |
012919EV01 |
020419SP01 |
060319BRSP01 |
|
Protein (dry basis) |
≥85% |
84.6 |
89.1 |
86.9 |
89.7 |
88.5 |
|
Moisture |
<8% |
4.5 |
2.9 |
4.4 |
4.3 |
5.1 |
|
Fat |
<2% |
0.26 |
0.84 |
1.26 |
1.00 |
0.45 |
|
Total carbohydrates |
<10% |
8.92 |
6.79 |
6.36 |
5.83 |
7.25 |
|
Total fibre |
N/A |
2.8 |
2.8 |
3.6 |
3.2 |
3.2 |
|
Soluble |
N/A |
2.0 |
2.0 |
3.1 |
2.3 |
2.8 |
|
Insoluble |
N/A |
0.8 |
0.8 |
0.5 |
0.9 |
0.4 |
|
Ash |
<8% |
5.51 |
2.97 |
4.85 |
3.06 |
3.37 |
N/A= not available
17. Barley Rice Protein contains an amino acid composition that is similar to the native composition of barley and rice (whilst accounting for natural variation). The applicant provided analytical data on 5 production batches of Barley Rice Protein; the results of which demonstrated that the production process applied yielded a consistent product that conforms to the established product specifications. In addition, analytical data were presented for potential chemical and microbiological impurities, mycotoxins and other secondary metabolites of concern. The methods of analysis used were internationally recognised (or equivalent) or were developed and validated internally by the applicant, as outlined below.
18. Heavy metals analysis by inductively coupled plasma mass spectrometry of 5 production batches of Barley Rice Protein was provided. This demonstrated that heavy metals, including arsenic, cadmium, lead and mercury were below established specification limits of 0.1 ppm for arsenic, cadmium, and mercury or 0.2 ppm for lead. The analytical results for cadmium and arsenic were below the limits established by Commission Regulation (EC) No 1881/2006 as retained in UK law setting maximum levels for certain contaminants in foodstuffs (Table 2).
Table 2. Results of heavy metal analysis of 5 non-consecutive batches of Barley Rice Protein
|
Parameter |
Specification Limit |
Manufacturing Lot Number: 080318SP01 |
122018BRSP01 |
012919EV01 |
060319BRSP01 |
070819BRSP01 |
|
Arsenic |
<0.1 ppm |
0.075 |
0.066 |
0.028 |
0.039 |
0.041 |
|
Cadmium |
<0.1 ppm |
0.025 |
0.033 |
0.021 |
0.024 |
0.021 |
|
Lead |
<0.2 ppm |
0.031 |
<0.010 |
0.015 |
0..012 |
0.016 |
|
Mercury |
<0.1 ppm |
<0.010 |
<0.010 |
<0.010 |
<0.010 |
<0.010 |
ppm = parts per million
19. Microbiological analysis of 4 production batches of Barley Rice Protein was also presented (Table 3). This demonstrated Barley Rice Protein met established microbiological specifications, as outlined in Table 3.
Table 3. Results of microbiological analysis of 4 non-consecutive batches of Barley Rice Protein
|
Parameter |
Specification Limit |
Manufacturing Lot Number: 080318SP01 |
122018BRSP01 |
012919EV01 |
020419SP01 |
|
Aerobic Plate Count |
<30,000 CFU/g |
5,700 |
6,000 |
4,300 |
7,100 |
|
Coliforms |
<10 CFU/g |
<10 |
<10 |
<10 |
<10 |
|
Yeast/Mould |
<50 CFU/g |
<10 |
<10 |
<10 |
<10 |
|
Salmonella |
Negative in 25g |
ND |
ND |
ND |
ND |
|
Escherichia coli |
<10 CFU/g |
<10 |
<10 |
<10 |
<10 |
|
Staphylococcus aureus |
<10 CFU/g |
<10 |
<10 |
<10 |
<10 |
|
Listeria spp. |
Negative in 25g |
Not tested |
ND |
ND |
ND |
CFU = colony-forming unit; ND = not detected
20. The applicant presented analyses for the presence of mycotoxins, such as aflatoxin B1, B2, G1, or G2, total fumonisins, T-2 toxin, HT-2 toxin, deoxynivalenol, or zearalenone for 5 production batches of Barley Rice Protein (Table 4). These mycotoxins were analysed using a combination of an internationally recognised method [i.e., Association of Official Analytical Chemists (AOAC 1999)] and internal method (LC-MS/MS) and were demonstrated to be below the detection limit across all 5 batches, suggesting the acceptable limit of these compounds in the final product.
21. Total aflatoxins (B1, B2, G1, G2) were below the limit of detection of 5 µg/kg, which is consistent with the limits for these mycotoxins as established by Commission Regulation (EC) No 1881/2006 as retained in UK law setting maximum levels for certain contaminants in foodstuffs. The assessment highlighted that in one batch the aflatoxin total level was <4 µg/kg, compared to <5 µg/kg for the other batches. Individual aflatoxin values were also lower for this batch compared to the other batches. The applicant clarified the same validated method was used for all analyses with an LOQ for the aflatoxins analyses of 1 µg/kg. Therefore, the reported result of <4 and <5 µg/kg are possible (result will depend on the dilution that was made for the analysis). Risk managers may wish to give further consideration to aflatoxin levels, for individual batches.
22. The ACNFP sought to understand why two results for batch 060319BRSP01 have not been measured. The applicant clarified there was no analyte recovery for Aflatoxins G1 and G2, and therefore could not be quantified for this batch. The applicant provided analyses for mycotoxins in 6 additional production batches of Barley Rice Protein demonstrating the levels to be below the LOQ and levels of aflatoxins consistently below the LOD of 5 µg/kg.
Table 4. Analysis for mycotoxins in 5 non-consecutive batches of Barley Rice Protein
|
Mycotoxin |
Method of Analysis |
Manufacturing Lot Number: 080318SP01 µg/kg |
122018BRSP01 µg/kg |
012919EV01 µg/kg |
060319BRSP01 µg/kg |
070819BRSP01 µg/kg |
|
Aflatoxin B1 |
AOAC 999.07 (modified) |
<5 |
<2 |
<5 |
<5 |
<5 |
|
Aflatoxin B2 |
AOAC 999.07 (modified) |
<5 |
<2 |
<5 |
<5 |
<5 |
|
Aflatoxin G1 |
AOAC 999.07 (modified) |
<5 |
<2 |
<5 |
NM |
<5 |
|
Aflatoxin G2 |
AOAC 999.07 (modified) |
<5 |
<2 |
<5 |
NM |
<5 |
|
Aflatoxins total |
---- |
<5 |
<4 |
<5 |
<5 |
<5 |
|
Ochratoxin A |
AOAC 999.07 (modified) |
<5 |
<5 |
<5 |
<2 |
<5 |
|
Total fumonisins |
AOAC 92(20),496 |
<30 |
<30 |
<30 |
<30 |
<30 |
|
T-2 Toxin |
LC-MS/MS |
<1 |
<1 |
<1 |
<1 |
<1 |
|
HT-Toxin |
LC-MS/MS |
<10 |
<10 |
<10 |
<10 |
<10 |
|
Vomitoxin (Deoxynivalenol) |
LC-MS/MS |
<10 |
<10 |
<10 |
<10 |
<10 |
|
Zearalenone |
LC-MS/MS |
<5 |
<5 |
<5 |
<5 |
<5 |
AOAC= Association of Official Analytical Chemists; LC-MS/MS= liquid chromatography tandem mass spectrometry; NM= not measured.
23. The ACNFP raised questions on the implications on the product's composition in respect to the variability in starting materials. The applicant provided further proximate analysis information (figure 1) and argued that despite the high degree of variability in the ratio or barley to rice, there are no appreciable differences in specific parameters of the starting materials.
24. The ACNFP sought to understand the impact on the final novel ingredient of changing the proportions of the starting material and whether this was appropriately reflected in the specification.
25. The applicant presented the amino acid profile of Barley Rice Protein (Table 5) produced with a 55% rice and 45% barley BSG (brewers spent grain), as well as 30% rice and 70% barley to demonstrate no significant differences in the amino acid profile of Barley Rice Protein produced with different ratios of barley and rice, as well as no significant differences in the amino acid profile of the different BSG. After further clarification to gain reassurance that there were no other starting materials included if the levels of barley or rice were low, the level of addition was changed to 30-70% barley and 70-30% rice to reflect the data presented.
Table 5: Amino acid profile of Barley Rice Protein and BSG with different barley/rice ratios
|
Amino Acid |
BSG (55% Rice/45% Barley) (%) |
Barley Rice Protein (55% Rice/45% Barley) (%) |
BSG (30% Rice/70% Barley) (%) |
Barley Rice Protein (30% Rice/70% Barley) (%) |
|
Aspartic acid |
8.36 |
9.90 |
8.34 |
9.13 |
|
Threonine |
3.77 |
4.04 |
3.69 |
3.70 |
|
Serine |
4..69 |
5.00 |
4.66 |
4.41 |
|
Glutamine/glutamic acid |
18.45 |
22.03 |
18.33 |
23.93 |
|
Glycine |
4.38 |
4.62 |
4.27 |
4.25 |
|
Alanine |
5.50 |
5.34 |
5.53 |
4.62 |
|
Valine |
6.32 |
6.11 |
6.21 |
5.64 |
|
Methionine |
2.45 |
2.16 |
2.52 |
1.90 |
|
Isoleucine |
4.59 |
4.36 |
4.46 |
4.11 |
|
Leucine |
8.77 |
8.07 |
8.63 |
7.43 |
|
Tyrosine |
4.38 |
4.71 |
4.17 |
4.07 |
|
Phenylalanine |
5.91 |
5.79 |
5.92 |
5.95 |
|
Lysine |
3.77 |
3.71 |
3.88 |
3.35 |
|
Histidine |
2.34 |
2.45 |
2.33 |
2.16 |
|
Arginine |
6.93 |
5.99 |
6.89 |
4.69 |
|
Proline |
7.14 |
8.23 |
7.18 |
10.47 |
|
Cysteine |
1.83 |
1.29 |
1.84 |
1.52 |
|
Tryptophan |
1.63 |
1.54 |
1.65 |
1.51 |
26. The ACNFP considered all the information provided by the applicant for the composition and concluded it was sufficient for characterising Barley Rice Protein and had no further safety concerns.
2.5 Stability
27. The accelerated stability of Barley Rice Protein was investigated with 3 production batches of Barley Rice Protein. Samples of Barley Rice Protein were stored in sample pots in a climate chamber at 40°C and 75% relative humidity for up to 24 weeks. The results indicated no significant changes in the proximate parameters of Barley Rice Protein when stored for up to 24 weeks under accelerated conditions. Listeria monocytogenes and Salmonella spp. were not detected in 25 g of the product throughout the study period. The accelerated stability results were used to support the product shelf-life of up to 24 months using the Arrhenius equation.
28. The ACNFP sought clarification that additional measures were considered in determining the product shelf-life of up to 24 months. The applicant provided analysis of a further accelerated stability study with one production batch of Barley Rice Protein. Analysis was presented for sensory properties, which were acceptable along with proximate parameters and microbial contaminants. The ACNFP noted fluctuations in the moisture content in proximate analysis results, which were difficult to interpret or explain. The applicant further explained the use of the Arrhenius equation in this case and its use in estimating shelf-life, based on results from accelerated stability studies.
29. The applicant also provided results for stability under the intended conditions of use. The stability, nutritional profile, and sensory profile (i.e., appearance, aroma, flavour, and mouthfeel) of a final product was tested. A plant-based beverage made from Barley Rice Protein was assessed for 21 days and 170 days after production following storage under refrigerated conditions (0.56 to 3.33°C). The base of the plant-based beverage is made from Barley Rice Protein and blended with ingredients such as fats, sugars, vitamins, and minerals to achieve a neutral flavour, creamy texture, and a desired nutrient profile with respect to added minerals (e.g., calcium) as compared to other plant-based beverages. For the evaluation, the beverage was pasteurised, homogenised, aseptically filled into bottles, and stored at refrigeration temperatures of 0.56 to 3.33°C using pilot scale manufacturing equipment to mimic conditions of the commercial manufacturing process.
30. The nutrient profile and microbiological results were presented on a per-serving basis and as-is basis (portion size beverage is available in) (Table 6). The results demonstrated little to no change in the nutrient composition of the beverage over 170 days, and no changes in microbiological contaminants were reported. For the sensory assessments, individuals from a Taste Panel evaluated the beverage samples after up to 170 days of refrigerated storage to identify any undesirable changes to the appearance, aroma, flavour, or mouthfeel of the product. The Taste Panel reported a slight decrease in the overall aroma and flavour intensities between Day 21 and 170; however, samples were determined to meet the acceptance criteria for the brand profile, and no negative changes were observed. The above results demonstrated that the nutrient and sensory profiles of the plant-based product tested, made from Barley Rice Protein, are maintained for up 170 days when processed under commercial conditions and stored under recommended conditions.
Table 6. Stability results of Barley Rice Protein in a plant-based beverage following refrigerated storage for 21 days and 170 days
Nutritional Parameters
|
Parameter |
21 days: Per Serving (g/serving)* |
21 days: As-is Basis |
170 days: Per Serving (g/serving)* |
170 days: As-is Basis |
|
Calories Calculated (kcal/100 g) |
86 kcal/serving |
36 |
84 kcal/serving |
35 |
|
Carbohydrates, Calculated (%) |
11.51 |
4.78 |
10.54 |
4.45 |
|
Crude Fat by Acid Hydrolysis (%) |
2.48 |
1.03 |
2.49 |
1.05 |
|
Protein (%) |
4.51 |
1.88 |
4.74 |
2.00 |
Microbiological Parameters
|
Parameter |
21 days: Per Serving (g/serving)* |
21 days: As-is Basis |
170 days: Per Serving (g/serving)* |
170 days: As-is Basis |
|
Aerobic plate count |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
|
Total coliforms |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
|
Yeast and mould |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
|
Salmonella spp. |
Negative in 25 g |
Negative in 25 g |
Negative in 25 g |
Negative in 25 g |
|
Escherichia coli |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
|
Clostridium perfringens |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
<10 CFU/g |
|
Listeria spp. |
Negative in 25 g |
Negative in 25 g |
Negative in 25 g |
Negative in 25 g |
CFU = colony-forming units, *One serving size is equivalent to 237 ml
2.6 Specification
31. The applicant provided a specification table for Barley Rice Protein (Table 7). Analytical data were generated on 5 production batches of Barley Rice Protein, the results from these analyses demonstrated that the production process for Barley Rice Protein yielded a consistent product that conforms to the established product specifications.
Table 7. Specifications for Barley Rice Protein
General description: Barley Rice Protein is an off-white powder, produced by concentration of proteins from a mixture of barley and rice from the mash step of beer production using a series of enzymatic hydrolysis and mechanical purification steps.
Chemical parameters
|
Specification parameter |
Specification limit |
Method |
|
Protein (dry basis) |
≥85% |
AOAC 990.03; AOAC 992.15 |
|
Moisture |
<8% |
AOAC 925.09 |
|
Carbohydrates |
<10% |
Calculated |
|
Fat |
<2% |
AOAC 996.06 |
|
Ash |
<8% |
AOAC 942.05 |
Heavy metals
|
Specification parameter |
Specification limit |
Method |
|
Arsenic |
<0.1 mg/kg |
AOAC 844-856 (modified) |
|
Cadmium |
<0.1 mg/kg |
AOAC 844-856 (modified) |
|
Lead |
<0.2 mg/kga |
AOAC 844-856 (modified) |
|
Mercury |
<0.1 mg/kg |
AOAC 844-856 (modified) |
Microbiological Parameters
|
Aerobic plate count |
<30,000 CFU/g |
AOAC 966.23 |
|
Coliforms |
<10 CFU/g |
AOAC 991.14 |
|
Yeast and Mould |
<50 CFU/g |
FDA BAM Chapter 18 |
|
Salmonella |
Negative in 25 g |
AOAC-RI 121501 |
|
Escherichia coli |
<10 CFU/g |
AOAC 991.14 |
|
Staphylococcus aureus |
<10 CFU/g |
AOAC 2003.07 |
|
Listeria spp. |
Negative in 25 g |
AOAC PTM 081401 |
AOAC= Association of Official Analytical Chemists; CFU= colony-forming units; FDA BAM= Food and Drug Administration Bacteriological Analytical Manual.
a The specification limit for lead was established at <0.2 mg/kg to be consistent with the lead limit for cereals and pulses established under the retained Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs.
32. The ACNFP considered the specification provided was appropriate for characterising Barley Rice Protein and did not raise any safety concerns.
2.7 History of Use
33. The applicant provided a literature search which identified studies reporting relevant safety outcomes for Barley Rice Protein. The applicant noted that the novel food ingredient is a mixture of barley protein and rice protein derived from their respective plant sources (H. vulgare and O. sativa, respectively) that have a recognised history of consumption in the global population. However, the novel food Barley Rice Protein has no history of use in the UK or EU.
34. The Committee did not raise any concerns relating to this section of the dossier.
2.8 Proposed Uses
35. Barley Rice Protein is intended to be used as a substitutional plant source of protein and has been compared to rapeseed protein for daily intake and role in the diet. The applicant stated that the proposed food uses of Barley Rice Protein will supplement, rather than fully replace, other balanced sources of dietary protein in the diet, such as from animal or dairy sources. The proposed food-uses of Barley Rice Protein matched with the FoodEx2 food categorisation system and the corresponding use-levels were presented in Table 8.
36. The applicant does not intend for the novel food to increase the protein daily intake of the UK population. The anticipated intake of Barley Rice Protein will be similar to those currently permitted in the UK and would not significantly increase the current consumption of plant-based protein in the UK population. The applicant suggested the anticipated intake of Barley Rice Protein could be estimated using a similar approach as rapeseed protein, which is currently authorised for use as “a vegetable protein source in foods except in infant formula and follow-on formula” with no limitations with respect to maximum permitted use levels.
Table 8. Proposed food categories and use levels of Barley Rice Protein based on the FoodEx2 food classification system
|
Food Category (as intended to be included in the Union List) |
FoodEx2 Group Namea |
FoodEx2 Group No. |
FoodEx2 Level |
Max. Barley Rice Protein (g/100 g or 100 ml)b,c |
|
Bakery products |
Bread and similar products |
A004V |
L2 |
15 |
|
---- |
Fine bakery waves |
A009T |
L2 |
15 |
|
Breakfast cereals (incl. bars) |
Breakfast cereals |
A00CV |
L2 |
30 |
|
Spreadable fats and dressings |
Margarines and similar |
A0F1G |
L3 |
10 |
|
---- |
Butter and margarine/oil blends |
A039F |
L4 |
10 |
|
Grain products and pastas |
Pastas and rice (or other cereal)-based dishes |
A040M |
L3 |
30 |
|
Snack foods |
Fried or extruded cereal, seed, or root-based products |
A0EZX |
L2 |
30 |
|
Jam, marmalade and other fruit spreads |
Fruit / vegetables spreads and similar |
A04MN |
L3 |
30 |
|
Candy / Confectionery |
Confectionery including chocolate |
A04PE |
L2 |
15 |
|
Dairy and dairy imitates |
Dairy imitates |
A0BXC |
L3 |
50 |
|
---- |
Milk and dairy products |
A02LR |
L1 |
50 |
|
Dessert Sauces and syrups |
Dessert sauces / toppings |
A046F |
L2 |
15 |
|
---- |
Syrups (molasses and other syrups) |
A033R |
L3 |
15 |
|
Meat imitates |
Meat imitates |
A03TE |
L3 |
30 |
|
Soups and soup mixes |
Soups (dry mixture uncooked) |
A0B9J |
L3 |
150d |
|
---- |
Soups (ready-to-eat) |
A041L |
L3 |
15 |
|
---- |
Stock cubes or granules (bouillon base) |
A043F |
L3 |
15 |
|
Savoury sauces |
Gravy Ingredients |
A043Q |
L3 |
10 |
|
---- |
Savoury sauces |
A16GK |
L3 |
10 |
|
---- |
Condiments (including table-top formats) |
A04QN |
L2 |
10 |
|
Legume-based spreads |
Hummus |
A03VN |
L5 |
30 |
|
Nut-based spreads |
Nut/seeds paste/emulsion/mass |
A0F0M |
L4 |
20 |
|
Energy drinks |
Energy drinks |
A03GA |
L4 |
90 |
|
Food and beverages intended for sportsmen |
Carbohydrate-rich energy food products for sports people |
A03RY |
L4 |
30 |
|
---- |
Protein and protein components for sports people |
A03SA |
L4 |
90 |
|
Meal replacements for weight control |
Foods for weight reduction |
A03RS |
L3 |
90 |
Incl.= including; Max.= maximum
a Proposed uses and use levels of Barley Rice Protein were matched with the FoodEx2 food categorisation system developed by EFSA
b Dilution factors were obtained from EFSA (2018b): Internal report on the harmonisation of dilution factors to be used in the assessment of dietary exposure.
c The maximum use level was applied in the assessment to account for the “worst-case” scenario.
d Accounting for a reconstitution factor of 10 for dry soup mixtures (the maximum use level of Barley Rice Protein is 15% in powder-based soups, as consumed).
37. The ACNFP requested information on the likely intake for proposed uses of the product, in order to evaluate whether the potential use of the product may result in a nutritional disadvantage for consumers.
38. The applicant responded by conducting an example exposure assessment using the food consumption data from the United Kingdom (UK) National Diet and Nutrition Survey (NDNS) Rolling Programme, Years 7 to 9, 2014 to 2017 (NatCen Social Research/MRC Elsie Widdowson Laboratory, 20191). This assessment focused on the two food groups with a high proportion of consumers, with high daily intakes, dairy imitates, and meat imitates.
39. Table 9 summarised the estimated consumer only intake of Barley Rice Protein on an absolute and body weight basis (g/person/day and g/kg bw/day) from the key categories assessed, dairy or dairy/meat imitates, in UK consumers. The percentage of consumers was high among all age groups evaluated, with greater than 96.8% of the population consuming these foodstuffs.
Table 9. Summary of the estimated daily intake of Barley Rice Protein from selected food categories (NDNS data, Years 7 to 9)
Consumer-only intake
|
Population Group |
Age Group (Years) |
Absolute (g/person/day): n |
Absolute (g/person/day): % |
Absolute (g/person/day): Mean |
95th Percentile |
Body Weight (µg/kg bw/day): n |
Body Weight (µg/kg bw/day): % |
Body Weight (µg/kg bw/day): Mean |
95th Percentile |
|
Young Children |
1.5 to 3 |
354 |
100% |
51.7 |
109.1 |
309 |
100% |
3.6 |
8.5 |
|
Children |
3 to 10 |
922 |
99.5% |
41.8 |
98.2 |
870 |
99.6% |
1.9 |
4.9 |
|
Female Teenagers |
11 to 18 |
380 |
96.8% |
27.3 |
70.1 |
363 |
97.2% |
0.5 |
1.3 |
|
Male Teenagers |
11 to 18 |
374 |
98.0% |
38.2 |
106.9 |
353 |
97.8% |
0.7 |
2.0 |
|
Female Adults |
19 to 64 |
896 |
99.2% |
38.5 |
93.5 |
825 |
99.2% |
0.6 |
1.4 |
|
Male Adults |
19 to 64 |
645 |
98.2% |
41.4 |
104.6 |
607 |
98.2% |
0.5 |
1.3 |
|
Elderly |
≥65 |
500 |
99.5% |
61.5 |
126.0 |
427 |
99.4% |
0.7 |
1.8 |
|
Total Population |
≥1.5 |
3926 |
98.8% |
43.7 |
104.6 |
3620 |
98.8% |
0.8 |
2.4 |
n = sample size; NDNS = National Diet and Nutrition Survey; UK= United Kingdom
2.9 Absorption, distribution, metabolism and excretion (ADME)
40. Barley Rice Protein is expected to be hydrolysed and digested in a similar manner as other dietary proteins, based on the similarity of composition with rice and barley. Studies on digestibility of cooked and uncooked barley and rice were provided as evidence by the applicant. These supported that Barley Rice Protein would be readily digested, yielding individual amino acids and small peptides that would be absorbed and handled by the body in normal metabolic processes, similar to that of other dietary protein sources. The applicant noted proteins that are readily digested due to denaturation and degradation processes along the gastrointestinal tract are not likely to pose a safety concern compared to proteins that are resistant to digestion. The Committee considered the information provided was satisfactory and did not request any further information for the ADME section.
2.10 Nutritional information
41. Information on proximate analysis of 5 different batches was provided by the applicant (Table 1). This analysis demonstrated Barley Rice Protein was primarily comprised of protein (>85%, dry solids) with the remaining components being ash (typically <8%), moisture (<8%), fat (typically <2%), carbohydrates (typically <10%) and fibre (typically <5%). To address the protein quality of Barley Rice Protein, the applicant provided data on the amino acid profile for four batches of Barley Rice Protein (Table 5). The applicant stated the amino acid profile of Barley Rice Protein is comparable to that of native barley and rice and confirmed that the manufacturing process did not chemically alter the starting material. The applicant does not expect the protein quality to pose a nutritional disadvantage compared to existing plant-based proteins (e.g., soy protein or rapeseed protein), due to the similarity in amino acid composition to those it is intending to replace in the UK marketplace (soy protein isolate and rapeseed proteins) (Table 10). Levels of protein, amino acids, vitamins, minerals, and fatty acids in Barley Rice Protein were within acceptable ranges and did not give rise to safety concerns, unless replacing animal protein.
Table 10. Amino acid composition of plant-based proteins (g/100g)
|
Amino Acid |
Soy Protein Isolatea (%) |
Canolab (%) |
Barley Rice Proteinc (%) |
|
Tryptophan |
1.12 |
na |
1.51 |
|
Threonine |
3.14 |
4.81 |
3.70 |
|
Isoleucine |
4.25 |
4.47 |
4.12 |
|
Leucine |
6.78 |
7.47 |
7.43 |
|
Lysine |
5.33 |
6.6 |
3.35 |
|
Methionine |
1..13 |
2.24 |
1.90 |
|
Cystine |
1.05 |
2.08 |
1.52 |
|
Phenylalanine |
4.59 |
4.67 |
5..95 |
|
Tyrosine |
3.22 |
3.19 |
4.07 |
|
Valine |
4.1 |
5.65 |
5.64 |
|
Arginine |
6.67 |
7.28 |
4.69 |
|
Histidine |
2.3 |
3.18 |
2.16 |
|
Alanine |
3.59 |
4.53 |
4.62 |
|
Aspartic acid |
10.2 |
7.79 |
9.13 |
|
Glutamic acid |
17.5 |
20.81 |
23.93 |
|
Glycine |
3.6 |
4.60 |
4.25 |
|
Proline |
4.96 |
6.22 |
10.47 |
|
Serine |
4.59 |
4.41 |
4.41 |
a USDA SR Legacy (USDA (U.S. Department of Agriculture), 2019. Search results for Soy protein isolate (fdc.nal.usda.gov). In: FoodData central. Data Type: SR Legacy; FDC ID: 174276; NDB Number: 16122. (FDC Published:4/1/2019)
b Canola proteins for Human Consumption: Extraction, Profile and Functional Properties (Tan et al., 2011: Tan SH, Mailer RJ, Blanchard CL and Agboola SO, 2011. Canola proteins for human consumption: extraction, profile, and functional properties. Journal of Food Science, 76, R16-R28.)
c As per Table 5
42. The applicant identified a conclusion by the NDA EFSA panel stating a total recommended level of protein intake at 2.2 g/kg body weight/day is considered safe. The ACNFP advised that the applicant reassess what the expected consumption of Barley Rice Protein would be per day in this context. The applicant responded by calculating an estimated expected consumption of approximately 0.4 g protein/kg body weight/day is consumed from processed foods. This was based on protein from processed foods, which are considered the “best” representative products of the use of protein isolates such as rapeseed protein, and soy protein which would also include Barley Rice Protein, which contribute approximately 18% to total protein intakes. Dietary exposure would be 0.4 g/kg body weight/day if 100% of processed protein was to originate from Barley Rice Protein, which the applicant does not expect to be the case.
43. The applicant noted presence of anti-nutritional factors (such as phytic acid, oxalic acid, trypsin inhibitors, and lectins) and claimed these would be removed during the production process. The ACNFP advised the FSA and FSS that clarification should be sought that anti-nutritional factors were effectively reduced to safe levels. The applicant responded by providing details of steps during the production process where anti-nutritional factors are reduced and demonstrated the effectiveness of the process in the production of Barley Rice Protein across 4 production batches (Table 11).
Table 11. Analysis for antinutrients across 4 production batches of Barley Rice Protein
|
Antinutrient |
Manufacturing Batch No. |
|||
|
---- |
030521BR-AVEa |
160221BR-AVEb |
21120BR-AVEa |
290421BR-AVEb |
|
Phytic Acid (%) |
0.15 |
<0.14 |
<0.14 |
NM |
|
Oxalic Acid (g/100 g) |
<0.02 |
<0.02 |
<0.02 |
NM |
|
Calcium Oxalate (g/ 100 g) |
<0.0285 |
<0.0285 |
<0.0285 |
NM |
|
Trypsin Inhibitor (TIU/g) |
3,300 |
NM |
<3,200 |
<2,600 |
|
Lectin (mg/g) |
<0.05 |
NM |
<0.05 |
<0.05 |
NM= Not measured; TIU= trypsin inhibitor units.
aThis batch contained barley/rice ratio of 70/30%
bThis batch contained barley/rice ratio of 60/40%
44. Clarification was sought on the regulatory limits used for the antinutrients analysed. The applicant noted that regulatory limits for the analysed antinutrients currently do not exist in the EU or UK. The OECD (Organisation for Economic Co-operation and Development) highlighted that barley and rice contain common antinutrients and levels of these compounds have not been present at unsafe levels in both barley and rice. At the low levels presented in Table 11, the risks from the presence of these anti-nutrients is expected to be low.
45. A study on protein digestibility using the Protein Digestibility-Corrected Amino Acid Score (PDCAAS) was presented in the application, based on this the applicant concluded that the novel food would be readily digested. Furthermore, a series of indicators were given to characterise the nutritional value and protein quality evaluation of Barley Rice Protein, based on the two components of the ingredient.
46. The ACNFP concluded that based on a total protein intake value of 2.2 g/kg body weight/day, Barley Rice Protein is not expected to be nutritionally disadvantageous, on the assumption 100% of processed protein intake is not expected to originate from Barley Rice Protein alone.
2.11 Toxicological information
47. The applicant followed a tiered approach to safety evaluation as defined by the International Life Sciences Institute (Delaney et al., 2008). According to this, traditional animal toxicology studies are not necessary: if there is a history of use of the ingredients in foods, if the ingredient is fully characterised, the nutritional implications of the ingredient are fully assessed, and if no biological effects are identified from clinical studies.
48. The applicant based on this guidance suggested that no further testing would be necessary. Both barley and rice have an established history of consumption in the human diet, globally and within the UK population. The novel food ingredient has been characterised with respect to its purity and potential chemical and microbiological hazards and nutritional information has been evaluated using the Codex approach (Food Chemical Codex, 2018).
49. The ACNFP concluded that no further toxicological assessment for Barley Rice Protein was required.
2.12 Allergenicity
50. The applicant identified food allergens present in barley and rice that cause IgE-mediated food allergies; literature was presented suggesting that the frequency of allergy to barley and rice varied amongst the populations studied, allergies to these substances were rare. It was noted that in line with Annex II of Regulation 1169/2011, the novel ingredient would be labelled as containing a cereal containing gluten to reflect the potential risk for those with Coeliac disease.
51. The allergenicity potential of barley and rice was investigated by searching the databases of known and putative allergens of AllergenOnline (Version 19; dated 10 February 2019) and the World Health Organization/International Union of Immunological Societies (WHO/IUIS) (FARRP, 2019; WHO/IUIS, 2020). The applicant performed a search of the list of known and putative allergens in the AllergenOnline database and listed several groups of proteins that have been identified and characterised as food allergens in barley (H. vulgare) including alpha-amylase inhibitor BMAI-1 precursor (Hor v 15), alpha-amylase (Hor v 16), beta-amylase (Hor v 17), gamma-hordein 3 (Hor v 20), profilin (Hor v 21), and lipid transfer protein (Hor v LTP) (FARRP, 2019).
52. The potential allergenicity of rice was searched in a similar manner using the AllergenOnline and WHO/IUIS databases. The search revealed a number of allergenic proteins present in rice (Oryza sativa) such as trypsin alpha-amylase inhibitors, beta-expansin, and profilin A.
53. The ACNFP required further information on the allergenicity profile of Barley Rice Protein, if it was a hydrolysate and consideration of the risk to coeliacs from consumption. The applicant stated the majority of peptides present in the starting material are digested into short peptides of low molecular weight, that were in the 500 Da to 3 kDa range. Furthermore, the applicant noted that the risk of cross-reactivity was low based on the minimum identity match to consider for possible cross-reactivity being 29 amino acids in any FASTA alignment, based on a 35% identity across an 80-amino acid length (Herman et al., 2009, Abdelmoteleb et al., 2021). This minimum amino acid length corresponds to an average molecular weight of 3.45 kDa, assuming an average molecular weight of 119 Da per amino acid residue, or between 2.2 and 5.9 kDa for the minimum and maximum molecular weights of 75.1 and 204.2 kDa for glycine and tryptophan, respectively.
54. The applicant noted that the majority of allergenic proteins in barley associated with coeliac disease are expected to be readily digested. However, since digestion products carry coeliac toxic motifs, the final product still presents a risk to individuals with coeliac disease.
55. The ACNFP concluded the potential allergenicity risk of Barley Rice Protein is not expected to be different from barley and rice allergenicity
3. Conclusions
56. The ACNFP have undertaken an assessment of Barley Rice Protein and concluded that they do not have any safety concerns relating to this novel ingredient.
57. Consumption of Barley Rice Protein would not be considered nutritionally disadvantageous if used alone or in combination with other plant sources of protein, however there are concerns it may be nutritionally disadvantageous if used as a meat or dairy substitute in meal replacement products.
58. These conclusions are based on the information in the applicant’s dossier, supplemented by additional information the applicant provided and could not have been reached without the data presented in the “Protein quality report of Barley Rice Protein” claimed as proprietary by the applicant.
59. With thanks to the members of the ACNFP during the course of the assessment who were; Dr Camilla Alexander White, Dr Anton Alldrick, Alison Austin, Dr Mark Berry, Professor Dimitris Charalampopoulos, Professor Susan Duthie, Professor Susan Fairweather-Tait, Professor Paul Frazer, Dr Hamid Ghouddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw Jones, Dr Ray Kemp, Dr Elizabeth Lund, Nichola Lund, Dr Rohini Manuel, Emeritus Professor Harry McArdle, Rebecca McKenzie, Professor Clare Mills, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield and Professor Bruce Whitelaw. Dr Anton Alldrick declared a historical conflict of interest with regards to Barley Rice Protein and did not contribute any comments to the discussion but was present as an observer.
4. References
Abdelmoteleb M, Zhang C, Furey B, Kozubal M, Griffiths H, Champeaud M and Goodman RE, 2021. Evaluating potential risks of food allergy of novel food sources based on comparison of proteins predicted from genomes and compared to www.AllergenOnline.org. Food and Chemical Toxicology 147, 111888 [13pp, plus supplementary tables]. DOI:10.1016/j.fct.2020.111888.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Commission Regulation (EC) No 1881/2006 (legislation.gov.uk)
Delaney B, Astwood JD, Cunny H, Conn RE, Herouet-Guicheney C, Macintosh S, Meyer LS, Privalle L, Gao Y, Mattsson J and Levine M (ILSI International Food Biotechnology Committee Task Force on Protein Safety), 2008. Evaluation of protein safety in the context of agricultural biotechnology. Food and Chemical Toxicology, 46, suppl 2, S71-S97.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012. Scientific Opinion on Dietary Reference Values for protein. EFSA Journal, 10, 2557 [66pp]. DOI:10.2903/j.efsa.2012.2557. Available online: EFSA Scientific opinion on dietary reference values (efsa.europa.eu)
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Food and Food Allergens), 2021. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/22831 (Revision 1), EFSA Journal 2021;19(3):6555, 27 pp
EUR-Lex, 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Available online: Commission regulation (EC) No 1881/2006 (legislation.gov.uk)
EUR-Lex, 2011. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Available online: Regulation (EU) No 1169/2011 (legislation.gov.uk)
FARRP (Food Allergy Research and Resource Program), 2019. AllergenOnline version 19: Home of the FARRP allergen protein database. University of Nebraska-Lincoln, Food Allergy Research and Resource Program (FARRP), Lincoln, NE. Available online: http://www.allergenonline.org (Released: February 10, 2019).
FCC (Food Chemical Codex), 2018. Enzyme preparations. In: Food Chemicals Codex, 11th edition. United States Pharmacopeial Convention (USP), Rockville, MD, 413-417.
Herman RA, Song P and Thirumalaiswamysekhar A, 2009. Value of eight-amino-acid matches in predicting the allergenicity status of proteins: an empirical bioinformatic investigation. Clinical and Molecular Allergy: CMA, 7, 9 [7pp, plus supplementary data]. DOI:10.1186/1476-7961-7-9.
JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. General specifications and considerations for enzymes used in food processing [prepared by the Committee at its sixty-seventh meeting (2006)]. In: Combined compendium of food additive specifications, Joint FAO/WHO Expert Committee on Food Additives, 67th meeting, June 20-29, 2006, Rome. Food and Agriculture Organization of the United Nations (FAO), Rome. FAO JECFA Monograph 3, 63- 67. Available online: General specifications and considerations for enzymes used in food processing (fao.org)
NatCen Social Research/MRC Elsie Widdowson Laboratory, 2019. National Diet and Nutrition Survey Years 1-9, 2008/09-2016/17. [data collection]. 15th Edition. UK Data Service. SN: 6533. Available online: NDNS Years 1-9 (beta.ukdataservice.ac.uk)
2014/424/EU: Commission Implementing Decision of 1 July 2014 authorising the placing on the market of rapeseed protein as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document C(2014) 4256). OJ L 196, 3.7.2014, 27-29. Available online: Authorising the placing on the market of rapeseed protein as a novel food (eurlex.europa.eu)
WHO/IUIS (World Health Organization/International Union of Immunological Societies), 2020. Allergen nomenclature [database]. Available online: http://www.allergen.org/index.php (Last accessed: November 12, 2020).
Abbreviations
| FSA | Food Standards Agency |
| FSS | Food Standards Scotland |
| ACNFP | Advisory Committee on Novel Foods and Processes |
| BSG | brewers spent grain |
| AOAC | Association of Official Analytical Chemists |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| LOQ | limit of quantification |
| LOD | limit of detection |
| UK NDNS | United Kingdom national diet and nutrition survey |
| ADME | Absorption, distribution, metabolism and excretion |
| USDA | United States, Department of Agriculture |
| NDA | Dietetic Products, Nutrition and Allergies Panel |
| EFSA | European Food Safety Authority |
| OECD | Organisation for Economic Co-operation and Development |
| PDCAAS | Protein Digestibility-Corrected Amino Acid Score |
| WHO/IUIS | World Health Organization/International Union of Immunological Societies |
| FARRP | Food Allergy Research and Resource Program |
| TIU | Trypsin inhibitor units |