ACNFP Advice on the safety of a cannabidiol (CBD) isolate as a novel food for use in a range of food categories including food supplements.
On this page
Skip the menu of subheadings on this page.Reference Number RP350
Regulated Product Dossier Assessment
Assessment finalised: 29th April 2024
Summary
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in February 2021 from Cannaray Brands Ltd. (“the applicant”) for the authorisation of cannabidiol (CBD) isolate as a novel food.
The novel food is a >98% pure form CBD isolate which is intended to be used as a food ingredient in food supplements, beverages, and confectionary for adults.
For CBD a provisional Acceptable Daily Intake (ADI) of 10 mg/day has been published by the FSA and was considered in assessing this novel food. The provisional ADI (section 2.7) was recommended, subject to the existing advice to consumers that pregnant and breastfeeding women and people taking any prescription medication should avoid the consumption of CBD. Consumers on regular medications should seek advice from a medical professional before using any type of CBD food product. In addition, children and prospective parents trying for a baby are advised against consumption of CBD, as are those who are immunosuppressed, due to remaining data gaps and residual uncertainties concerning the safety of CBD for these groups of consumers. These contraindications would also apply to this novel food.
To support the FSA and FSS in their evaluations of the application, the Advisory Committee on Novel Foods and Processes (ACNFP) were asked to review the safety dossier and supplementary information provided by the applicant. Please note, the Committee did not consider any potential health benefits or claims arising from consuming the food, as the focus of the novel food assessment is to ensure the food is safe, and not putting consumers at a nutritional disadvantage.
The Committee concluded that the applicant had provided sufficient information to assure the novel food, a CBD isolate as outlined in application RP350, was safe under the proposed conditions of use. The anticipated intake levels and the proposed use of this pure form of CBD in foods and food supplements was not considered to be nutritionally disadvantageous.
1. Introduction
1. The ACNFP assessed the food safety risks of CBD isolate and its production under the proposed uses, in line with Article 7 of assimilated Commission Regulation (EU) 2017/2469. The regulatory framework and the technical guidance put in place by the European Food Safety Agency (EFSA) for full novel food applications is retained as the basis and structure for the assessment (EFSA NDA Panel, 2016).
2. An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in February 2021 from Cannaray Brands Ltd. (“the applicant”) for the authorisation of cannabidiol (CBD) isolate as a novel food. The novel food is a >98% pure form CBD isolate which is intended to be used as a food ingredient in food supplements, beverages, and confectionary for adults.
3. Advice was sought from the joint Subgroup of the ACNFP and the Committee on Toxicity (COT) on CBD and hemp derived products, on the quality of the toxicological evidence submitted to support the application. The ACNFP and COT have issued a joint statement on a provisional ADI that can be applied to CBD ingredients containing 98% or more CBD. This, and wider evidence available in the public domain, was taken into account in reviewing the toxicological evidence for this application.
4. The final advice from the Committee was agreed at the 164th meeting, allowing the FSA and FSS to complete the risk assessment.
5. This document outlines the conclusions of the ACNFP on the safety of a >98% pure form CBD isolate (as detailed in application RP350), as a novel food.
2. Assessment
2.1 Identity of Novel Food
6. The novel food is a CBD isolate and is a white crystalline powder of purity equal to or greater than 98%. Information to support this characterisation was provided for five batches of the novel food. CBD is found in natural form from hemp as the (-)- trans isomer.
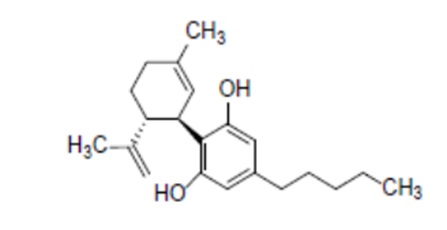
7. CBD in this application is characterised by the chemical formula: C21H30O2; molecular mass: 314.46 g/mol; CAS number: 13956-29-1; isomer: (-)-trans-cannabidiol; IUPAC name: 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol.
8. Confirmation of its identity was provided by Gas Chromatography – Flame Ionisation Detection (GC-FID), High Performance Liquid Chromatography – Ultra Violet (HPLC-UV), Gas Chromatography – Mass Spectrometry (GC-MS) (Electron impact ionisation) and Fourier-Transform Infrared Spectroscopy (FTIR).
2.2 Production Process
9. The CBD isolate is manufactured from industrial hemp using a multi-step extraction process under controlled conditions.
10. Certificates of analysis for raw starting materials used in the extraction process were provided to demonstrate the effectiveness of the controls at this point in the process. The details of the commercially sensitive extraction process were shared and reviewed by the ACNFP.
11. The industrial hemp is first tested to ensure it meets all internal specifications and regulatory requirements before it is accepted. Manufacturing begins with botanical raw material in the form of chipped or destemmed material. It is then extracted using ethanol and winterised/ fractionated to produce a crude extract and further refined to make a higher-potency distillate. The distillate then goes through several crushing, washing, distilling, and filtering steps to produce the highly purified CBD isolate.
12. The ACNFP considered whether the use of solvents as processing aids left any residues that needed to be flagged to risk managers. Comparison was made to residue limits for other consumed products as detailed in Table 1. Residues of solvents have been included in the specification.
Table 1: Comparison of information on permitted residue levels for solvents used in the novel foods production compared to the proposed specification.
|
Solvent used |
Available data on safe maximum level of consumption |
Level in specification for the novel food |
|
Ethanol |
Guidance on residues in Pharmaceutical products states it to be a class 3 solvent which should be limited by GMP or other quality-based requirements. 50 mg per day or less (5000 ppm) would be acceptable without justification1. |
45.8 mg/kg CBD |
|
Pentane |
Guidance on residues in Pharmaceutical products states it to be a class 3 solvent which should be limited by GMP or other quality-based requirements. 50 mg per day or less (5000 ppm) would be acceptable without justification1
|
45.8 mg/kg CBD |
1 ICH guideline Q3C (R8) on impurities: guideline for residual solvents.
13. The evidence presented (see Table 2 below) on composition indicates compliance with the specification for residues of solvents. When considered at the level of consumption the evidence suggests the levels of solvent residues in the novel food are below those which would represent a safety concern.
14. A HACCP statement was provided along with further details of the process and how it operates. The production process has characterised the potential hazards and detailed the corresponding control measures sufficiently.
2.3 Compositional Information
15. Results from five independent batches of the novel food demonstrated that the CBD content is produced consistently (Table 2).
Table 2: Compositional analysis of representative batches of cannabidiol (CBD) isolate
|
Parameter |
Method of Analysis |
Specification/ LOQ |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
|
Colour and Appearance |
Visual |
White crystalline powder free of particulates |
Conforms |
Conforms |
Conforms |
Conforms |
Conforms |
|
CBD Identification |
Chromatographic and Spectral analysis |
Analytical Reference Standard |
Corresponds to reference |
Corresponds to reference |
Corresponds to reference |
Corresponds to reference |
Corresponds to reference |
|
CBD content (% w/w) |
HPLC-DAD1 |
0.03 |
99.58 |
99.60 |
100.03 |
100.08 |
100.23 |
|
THC (% w/w) |
HPLC-DAD1 |
0.004 |
ND |
ND |
ND |
ND |
ND |
|
THCA (% w/w) |
HPLC-DAD1 |
0.032 |
ND |
ND |
ND |
ND |
ND |
|
CBN (% w/w) |
HPLC-DAD1 |
0.018 |
ND |
ND |
ND |
ND |
ND |
|
d8-THC (% w/w) |
HPLC-DAD1 |
0.056 |
ND |
ND |
ND |
ND |
ND |
|
THCV (% w/w) |
HPLC-DAD1 |
0.042 |
ND |
ND |
ND |
ND |
ND |
|
CBC (% w/w) |
HPLC-DAD1 |
0.032 |
ND |
ND |
ND |
ND |
ND |
|
CBDA (% w/w) |
HPLC-DAD1 |
0.026 |
ND |
ND |
ND |
ND |
ND |
|
CBDV (% w/w) |
HPLC-DAD1 |
0.038 |
0.43 |
0.43 |
0.43 |
0.43 |
0.43 |
|
CBGA (% w/w) |
HPLC-DAD1 |
0.030 |
ND |
ND |
ND |
ND |
ND |
|
CBG (% w/w) |
HPLC-DAD1 |
0.060 |
ND |
ND |
ND |
ND |
ND |
|
Total Cannabinoids (CBD plus related, % w/w) |
HPLC-DAD1 |
------------- |
100.01 |
100.03 |
100.46 |
100.51 |
100.66 |
|
Residual Solvent- Ethanol (ppm) |
HS-GC-MS2 |
45.8 ppm |
ND |
ND |
ND |
ND |
ND |
|
Residual Solvent- Pentane (ppm) |
HS-GC-MS2 |
45.8 ppm |
ND |
ND |
ND |
ND |
ND |
|
Parameters |
Method of Analysis |
Specification/ LOQ |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
|
Pesticides |
GC-MS/MS3 |
Individual pesticide limit ≤LOQ |
≤LOQ |
≤LOQ |
≤LOQ |
≤LOQ |
≤LOQ |
|
Elemental Impurities (Arsenic, ppm) |
AOAC 2011.19 / modified 993.14 |
0.01 |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
|
Elemental Impurities (Cadmium, ppm) |
AOAC 2011.19 / modified 993.14 |
0.005 |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
|
Elemental Impurities (Lead, ppm) |
AOAC 2011.19 / modified 993.14 |
0.005 |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
|
Elemental Impurities (Mercury, ppm) |
AOAC 2011.19 / modified 993.14 |
0.005 |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
|
Aflatoxins (ppb) |
USP<561> |
4.0 |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
|
Ochratoxin A (ppb) |
USP<561> |
2.0 |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
<LOQ |
LOQ = Limit of quantitation; ND = Not detected HS = Headspace; GC = Gas chromatography; MS/MS = tandem mass spectrometry; AOAC = Association of Official Analytical Chemists; NM = not measured; USP = United States Pharmacopeia
1HPLC-DAD = This method for the quantitation of 11 phytocannabinoids in Cannabis concentrates was followed by using protocols on statistic analytical analyses and reference article Fast Detection of 10 Cannabinoids by RP-HPLC-DAD Method in Cannabis sativa L. The method parameters were subject to AOAC SMPR 2017.001 and guidance from AOAC SMPR 2018.10.
2HS-GC-MS = This method for determination and quantitation of residual solvents in phytocannabinoid concentrates was developed and evaluated by the applicant. This method was validated by following ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1) and met the requirements provided in AOAC SMPR® 2019.002.
3GC-MS/MS = This method for the qualitative and quantitative analysis of pesticides by GC-MS/MS was developed with reference to the Shimadzu paper. The method also referenced AOAC Official Method 2007.01
16. CBD content is consistently above 98% pure with negligible amounts of other cannabinoids detected across the five representative batches.
17. It is recognised that the detection and characterisation of cannabinoids in a range of food matrices is an evolving area and there are yet to be internationally recognised methods. The limitations of analytical methodology available have been subject to discussion in the Joint ACNFP and COT CBD Subgroup and remains a source of uncertainty in the assessment. As a result, the robustness, accuracy and precision of the methods have been considered in interpreting the data on THC and were considered appropriate in this case.
18. Delta-9-tetrahydrocannabidiol (THC), as a potential contaminant in the novel food, was declared as not detected in any of the five batches tested (Table 2), with a limit of detection of 0.002% (w/w) in the analytical method used.
19. A literature review was undertaken as part of the assessment of CBD as a novel food, to understand the impact on the safety of foods with trace levels of contamination with THC. The joint ACNFP and COT subgroup reviewed the information from literature and identified a point of departure from the European Food Safety Authority (EFSA) opinion on THC as a contaminant in milk and meat (EFSA,2015). The point of departure was a LOAEL (Lowest Observed Adverse Effect Level) of 0.036mg/kg/bw/day from the most sensitive individuals and the lowest dose tested in the human clinical studies reviewed.
20. Uncertainty factors, including a factor of 3 to extrapolate from a LOAEL to a NOAEL (No Observed Adverse Effect Level), were applied to the LOAEL identified. This was considered appropriate as the effects are mild to moderate in severity. A further factor of 10 was applied for person-to-person variation, resulting in an uncertainty factor of 30. The factor of 30 applied to the 0.036mg/kg/bw/day LOAEL results in a level of 1µg /kg bw/day that would be represent a safe upper intake for consumed THC as a contaminant in food. This was identified an acute reference dose (ARfD) (EFSA, 2015)
21. The Subgroup agreed the ARfD to be sufficiently protective to apply to the UK population. It was noted that in applying the acute reference dose EFSA have assumed that the effects seen would be the same if humans were exposed to multiple doses of THC at very low levels. The Subgroup commented that there was no data to verify this assumption, but if setting limits the dataset is the best available.
22. The levels of THC in the novel food, once adjusted to take into account the proposed uses - 10mg of CBD being consumed a day-, were below the ARfD identified by EFSA of 1µg /kg bw/day or 70µg/day for a healthy adult. This level does not present a concern in terms of consumer safety for the novel food under the proposed conditions of use.
23. To ensure THC levels remain consistently low in the production of CBD, THC should be a standard substance included in the specification as relevant to all batches produced.
24. Analytical data, presented for five independent batches of the novel food (Table 2), demonstrated the levels of heavy metals where detected were present in very low levels and below established UK regulatory limits where applicable (applicable for Arsenic, Cadmium, Mercury and Lead; Table 3).
Table 3: Elemental Impurities in the novel food
|
Parameter1 |
Specification (ppm) |
|
Arsenic |
< 1.0 |
|
Cadmium |
< 0.4 |
|
Lead |
< 0.5 |
|
Mercury |
< 0.2 |
1=AOAC Official Method 2011.19 and 993.14 (modified) using Inductively Coupled Plasma/Mass Spectrometry; (ICP-MS)
25. Analytical data concerning the microbiological content from one batch of the novel food was reported (Table 4). The process in manufacturing this novel food uses extreme high and low temperatures and alcohol solvents. Full microbial risk assessment as per USP 2022 and 2021 confirm that the novel food shows absence of microbial growth.
Table 4: The microbiological analysis of the novel food (CBD isolate)
|
Parameter |
Result |
|
Escherichia coli (E. coli) |
Absent / 10 g |
|
Salmonella USP |
Absent / 10 g |
|
Staphylococcus aureus |
Absent / 10 g |
|
Aerobic Plate Count |
< 100 CFU/g |
|
Yeast Count |
< 100 CFU/g |
|
Mould Count |
< 100 CFU/g |
26. Additionally, water activity as measured in one batch of CBD isolate was considered. CBD isolate has a water activity of ~0.458 suggesting that it will not support microbial growth.
Table 5: Water activity analysis of the novel food (CBD isolate)
|
Sample |
Result (aw) |
|
CBD Isolate |
0.458 |
27. The data presented did not indicate any additional hazards for inclusion in the specification. Therefore, microbial analysis is not a required parameter for inclusion in the specification of the CBD isolate.
2.4 Stability
28. The stability of the CBD isolate was assessed under real-time conditions (25 oC and 60% relative humidity) in five batches for 24 months. Results showed that the novel food meets the specification criteria for CBD content, and no changes in appearance, water content and impurity levels are seen over these time periods.
29. The stability of the CBD isolate was assessed under accelerated conditions (40 oC and 75% relative humidity) in five batches for 6 months. Results confirmed that the novel food meets the specification criteria for CBD content and no changes in appearance, water content and impurity levels are seen over these time periods. The THC content was also tested and no significant changes in the levels of THC were observed.
30. The data provided supports the stability of CBD isolate for a period of at least 24 months.
2.5 Specification
31. The specification parameters reported in Table 6 were assessed using internationally recognised methods or determined using internally developed and validated methods. The results of the analysis are detailed in Table 2 and indicate the novel food can be produced consistently to the specification.
Table 1: Specification of the novel food
|
Description |
|
Cannabidiol is a white crystalline powder free of particulates produced by a multistep extraction process |
|
Parameter |
Specification |
Method |
|
Appearance |
White, crystalline powder free of particulates |
Visual inspection |
|
Identity |
Complies |
DAD Retention Time, DAD UV Spectrum: in house method |
|
CBD (% w/w) |
≥98% |
HPLC-DAD: validated in house method |
|
THC (Δ9-Tetrahydrocannabinol) (% w/w) |
≤ 0.004 |
HPLC-DAD: validated in house method
|
|
THCA (Δ9-Tetrahydrocannabinolic Acid) (% w/w) |
≤ 0.032 |
HPLC-DAD: validated in house method
|
|
Total Related Cannabinoid Content (% w/w) |
Report1 |
HPLC- DAD: validated in house method |
|
Residual solvents (ppm) |
------------- |
-------------- |
|
Pentane |
≤1000 |
HS-GC-MS: validated in house method |
|
Acetone |
≤1000 |
HS-GC-MS: validated in house method |
|
Isopropyl Alcohol (2-propanol) |
≤1000 |
HS-GC-MS: validated in house method |
|
Ethanol |
≤1000 |
HS-GC-MS: validated in house method |
|
Ethyl Acetate |
≤1000 |
HS-GC-MS: validated in house method |
|
Hexane |
≤60 |
HS-GC-MS: validated in house method |
|
Benzene |
≤1 |
HS-GC-MS: validated in house method |
|
Individual Pesticide Content (ppm) |
Individual Pesticide Limit2 |
GC-MS/MS: validated in house method |
|
Elemental Impurities (ppm) |
------------ |
--------------- |
|
Arsenic |
< 1.0 |
ICP-MS3 |
|
Cadmium |
< 0.4 |
ICP-MS3 |
|
Lead |
< 0.5 |
ICP-MS3 |
|
Mercury |
< 0.2 |
ICP-MS3 |
|
Mycotoxins (ppb) |
------------ |
-------------- |
|
Aflatoxin |
≤ 4.0 |
USP <561> |
|
Ochratoxin A |
≤ 2.0 |
USP <561> |
CBD = cannabidiol; GC = gas chromatography; HPLC = high-performance liquid chromatography; IR = infrared spectroscopy; USP = United States Pharmacopeia; DAD = diode-array detection; HS-GC-MS = headspace gas chromatography-mass spectrometry; ICP-MS = inductively coupled plasma mass spectrometry; GC-MS/MS = gas chromatography tandem mass spectrometry, 1= LOQ and LOD values for cannabinoids found in Table 2.4.1-7 in application, 2= Individual pesticides and associated limits found in Table 2.5.4-1 in application, 3=AOAC 2011.19 / modified 993.14
32. The ACNFP concluded the information provided is sufficient for the specification of CBD and appropriately characterises the novel food seeking authorisation.
2.6 History of Use
33. Hemp has been widely consumed in the UK and EU as a seed oil, in tea and as an alternative to hops in beer. Extracts of hemp including CBD and synthetic CBD have not been widely consumed and are considered novel foods. While CBD products are widely available on the UK high street, indicating some consumption of CBD as a food, at the time of publication, no previous applications for CBD have yet received authorisation as a novel food.
34. As detailed in the COT review of the literature there has been use of both hemp derived and synthetic forms of CBD for medicinal purposes. These provide an indication of the toxicological effects that should be explored in the testing regime – primarily effects on liver, thyroid and potential impacts on reproductive organs. Also reported are behavioural effects such as somnolence (sleepiness).
35. As reported in the COT review of the publicly available data on CBD and summary data on a medicinal product, signs of adverse effects on the liver were observed at doses of CBD as low as 5 mg/kg bw/day in patients and healthy human volunteers; this dose is equivalent to 350 mg in a 70 kg adult. The data in the literature also suggested that humans might be more sensitive to the adverse effects of CBD in the liver than laboratory animals.
36. Somnolence effects were noted at doses ≤10 mg/kg bw/day in human studies. Inhibitory drug-drug interactions have also been observed with some medications when CBD is co-administered at doses of 1 mg/kg bw/day (equivalent to 70 mg in a 70 kg adult); the likelihood of effects at lower doses has not been determined.
37. It is noted that the doses of CBD used for medicinal purposes are higher than those proposed for food use. The purpose of an assessment for medicines authorisation is different to that for food and this is reflected in the data requirements. Unlike medicines, there is no risk-benefit context in foods with the requirement instead being that the products are safe. This means that outcomes that are considered to be an adverse event for food might be weighed differently in the context of the clinical benefits in a medicinal study.
38. Within the literature, further human studies utilising chemically derived CBD provide further evidence of a history of synthetic CBD use (Izegelov et al., 2010; Stereo Biotechs Ltd., 2020; Klotz et al., 2019; Wheless et al., 2019). A review by Heuestis et al., 2019 of cannabidiol adverse effects and toxicity notes that, while CBD is not risk-free, severe adverse events occur at doses higher than those recommended for human pharmacotherapies which are prescribed to treat forms of epilepsy. The data on previous consumption of CBD suggest areas for careful consideration in the toxicological review to understand potential effects at the lower doses used in foods.
2.7 Proposed Use and Anticipated Intake
39. The intended use is food supplements as defined by UK legal requirements (The Food Supplements (England, Scotland and Wales) Regulations 2003) in a range of forms and a range of food and beverages. The joint ACNFP and COT statement on >98% pure form CBD in novel foods identified a provisional ADI which is equivalent to 10 mg per day for a healthy adult. In light of the new FSA consumer advice (published in October 2023), the applicant has amended the proposed use levels for both food supplements and food and beverages. These are detailed in Table 7. The provisional ADI is discussed in the Toxicological information section.
Table 7: Amended proposed uses and maximum use levels for the novel food.
|
Food category |
Maximum use level per day (mg CBD/day) |
|
Cocoa and Chocolate products as covered by Directive 2000/36/EC |
10 |
|
Other confectionery with added sugar |
10 |
|
Other confectionery without added sugar |
10 |
|
Chewing gum with added sugar |
10 |
|
Chewing gum without added sugar |
10 |
|
Water, including natural mineral water as defined in Directive 2009/54/EC and spring water and all other bottled or packed waters |
10 |
|
Fruit juices as defined by Directive 2001/112/EC 0.43 30 |
10 |
|
Flavoured drinks with sugar |
10 |
|
Flavoured drinks with sweetener |
10 |
|
Food supplements as defined in directive 2002/46 |
10 |
|
Food Supplements (for adults)) as defined in the Food Supplements (England) Regulations 2003 capsules and tablets and similar forms, excluding. chewable forms intended for those 18 years of age or older |
10 |
|
Food supplements (for adults)) as defined in the Food Supplements (England) Regulations 2003 supplied in a liquid form intended for those 18 years of age or older |
10 |
|
Food supplements (for adults) ) as defined in the Food Supplements (England) Regulations 2003 supplied in syrup or chewable form intended for those 18 years of age or older |
10 |
40. It is noted that consumers may be exposed to CBD from a range of food categories. The standard methodology for calculating exposure for a novel food would explore intake from a range of sources and ensure that exposure via the proposed uses would not exceed any safety level identified when consumption of the food category was analysed. It is noted that for CBD that there are already many products available. As such, the assessment has been made on the basis of the provisional ADI for CBD at 10 mg per day, which is a dose that can be interpreted as safe and would not be expected to lead to any adverse effects in consumers. As such proposed uses will only be considered as safe for all consumers within the assessment when below a maximum intake of 10mg of CBD per day.
41. Risk managers must consider whether consumers would benefit from information on the CBD content of foods in order to ensure their consumption does not exceed the provisional ADI of 10mg per day for a healthy adult.
42. As recommended in the ACNFP and COT statement on CBD of > 98% purity, “The provisional ADI is recommended, subject to the existing advice to consumers that pregnant and breastfeeding women and people taking any prescription medication should avoid the consumption of CBD. Consumers on regular medications should seek advice from a medical professional before using any type of CBD food product. In addition, children and prospective parents trying for a baby are advised against consumption of CBD, as are those who are immunosuppressed, due to remaining data gaps and residual uncertainties concerning the safety of CBD for these groups of consumers.”
43. The food supplement products are to be labelled in accordance with the labelling requirements of Food Supplements (England) Regulations 2003 as follows: Does not exceed the safe limit of 10 mg/day for a 70kg healthy adult. The novel food authorisation should also include the following: Not suitable for use under the age of 18. Not suitable for use during pregnancy or breastfeeding. If you are taking medication or have existing health conditions, please consult your doctor before using this product.
44. The ACNFP explored the potential for foreseeable misuse of the novel food. It is highlighted to risk managers that they may wish to consider whether risk management measures are needed beyond those in the food supplements regulation to ensure consumers are aware of the maximum safe dose for CBD intake per day and the excluded groups. The ACNFP also strongly recommended that risk managers consider how consumers can be supported to manage their intake appropriately within the safe limits identified and appreciate the nature of the potential risks at higher doses, for uses that are not in dosed forms.
2.8 Absorption, Distribution, Metabolism and Excretion (ADME)
45. The Absorption, Distribution, Metabolism and Excretion (ADME) of cannabidiol are known to be complicated by the food matrix in which the CBD is delivered and are currently still being defined by professional bodies.
46. The oral bioavailability of CBD is low, indicating that it is not absorbed to any notable extent following ingestion (Mechoulam et al., 2002). Published works report the bioavailability of CBD to be between 13 and 19% (Grotenhermen, 2003) or 6% (Hawksworth and McArdle, 2004). The low systemic availability was demonstrated by Martin-Santos et al., (2012) and further supported by a literature search which identified the pharmacokinetics of CBD in humans and concluded with dose dependent peak plasma concentrations of CBD and area under the curve results indicating minimal accumulation (Miller et al., 2018).
47. Following oral consumption, CBD is extensively metabolised in the liver. This rapid first pass metabolism contributes to the low oral bioavailability reported in the literature (Taylor et al., 2018; WHO, 2018). In vitro studies indicate that CYP3A4 and CYP2C19 are the primary hepatic enzymes responsible for first-pass metabolism of CBD; however, several other hepatic cytochrome P450 isoforms (CYP1A1, CYP1A2, CYP2C9, CYP2D6, and CYP3A5) have also demonstrated a capability of metabolising CBD (Jiang et al., 2011; Zendulka et al., 2016).
48. The metabolism of CBD is thought to follow two separate pathways. One is P450-mediated, in which CBD is metabolised into its major metabolite 7-COOH-CBD (which is a chemically inactive compound). This is followed by further metabolic reactions which yield the minor metabolites of CBD, including 6-OH-CBD (Devinsky et al., 2018; Taylor et al., 2018;). The other involves decarboxylation (Kraemer et al., 2019). The resultant metabolites are predominantly excreted in faeces and urine (Hawksworth and McArdle, 2004; WHO, 2018).
49. Accumulation of CBD in plasma of up to 2-fold has been reported when steady state levels are compared with a single dose (such as in Taylor et al., 2018). Additionally, minimal evidence of plasma accumulation has been reported in dosing studies over 5–9 days (Millar et al., 2018; Sellers et al., 2013; Stott et al., 2013).
50. The pharmacokinetics of CBD (24 studies) have also been systematically reviewed by Millar et al., (2018)., most of which assessed the administration of CBD at doses of 5–20 mg/day. This correlates to a low dose application similar to this CBD novel food application. With oral administration, single doses of 5.4 and 10 mg CBD achieved peak serum concentrations (Cmax) of 0.9 and 2.5 ng/ml. The time to maximum concentration (Tmax) was approximately 1 h, with a half-life between 1 and 3 hours. Given the intended use of this CBD, with an approximate half-life of one to three hours, with a total clearance of six hours, there are no significant concerns of accumulation.
51. Based on the information on ADME it is noted that, the bioavailability of CBD is typically low but can be affected by food matrix. This provides context for interpreting the toxicological data. It was noted that the potential for CBD to accumulate in the body has not been examined based on the available data reviewed. This also suggested the food context for the novel ingredient could impact whether the CBD present in the ingredient is more or less bioavailable. This has been taken into account in considering the assessment factors to account for uncertainty in setting the provisional ADI.
2.9 Nutritional information
52. The ACNFP sought clarification of the potential for the presence of antinutritional factors from the preparation. It was noted that hemp can contain a range of substances that could impact the digestion and absorption of nutrients from the diet. These include phytic acid (which can negatively affect the bioavailability of dietary and endogenous minerals and proteins), tannins (which can interrupt the absorption of iron), trypsin Inhibitors (which can affect protein digestion), and saponins (which at larger quantities cause gastric irritation).
The product is highly purified as indicated in the information on the composition. There is no presence of other components that would impact the digestion or absorption of nutrients from the diet.
53. The data on nutritional composition confirms that CBD has no caloric or nutritional value. The application is not intending that CBD replace another food in the diet. Consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
2.10 Toxicological information
54. Toxicological studies on CBD were performed by the applicant to support the safety assessment of the novel food. The respective study reports are unpublished and claimed as confidential and proprietary data. They were considered essential in the assessment of the safety of the novel food and were reviewed by the ACNFP. How data on systemic toxicity was managed and interpreted in the context of the provisional ADI is explained in the subchronic toxicology section below.
2.10.1 Genotoxicity
55. In vitro genotoxicity testing of CBD was conducted under Good Laboratory Practice (GLP) conditions and utilised the following OECD guidelines: in vitro bacterial reverse mutation test (OECD TG 471) and in vitro mammalian cell micronucleus test (OECD TG 487). This approach is recommended by the UK Committee on Mutagenicity and is also the basis of guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283.
56. The in vitro bacterial reverse mutation test demonstrated that this CBD ingredient was non-mutagenic, in the absence or presence of metabolic activation. In addition, the in vitro mammalian cell micronucleus test demonstrated that CBD was non-clastogenic and non-aneugenic in the absence and presence of metabolic activation.
57. The results from these in vitro studies support the conclusion that the novel food (>98% pure CBD) is not genotoxic. This is consistent with the view of the Committee on Mutagenicity in reviewing CBD generically as a substance from evidence available in the public domain (Committee on Mutagenicity; MUT/MIN/2020/1, 2020).
2.10.2 Sub-chronic toxicology study
58. The joint subgroup of the ACNFP and COT was formed to address a series of questions in relation to the safety of CBD, cannabinoids and hemp-derived ingredients.
59. A weight of evidence approach has allowed the Subgroup to identify a provisional ADI for CBD ingredients of >98% purity of 0.15 mg/kg bw/day or 10 mg per day for a 70 kg healthy adult (Joint position paper from the ACNFP and COT; FSA consumer advice published in October 2023). This value was identified to be protective of the most sensitive known effects in the liver and thyroid parameters and included consideration of data gaps and uncertainties.
2.10.3 Sub-chronic data supplied in this application
60. This applicant provided a Repeated Dose 90-Day Oral Toxicity Study in Rodents [(ERBC study n. A4292)], which was conducted under GLP conditions and to OECD Technical Guideline 408. In this 90-day study, each group comprised 10 female and 10 male rats which were dosed with 0 (control – corn oil), 50, 100 or 150 mg/kg bw/day CBD once per day by oral gavage at a dose volume of 2 mL/kg body weight/day.
61. Review of the study supported the conclusion that it was of sufficient quality to support the safety of the novel food. The findings of the study were consistent with those considered in the development of the provisional ADI. It was, therefore, considered scientifically appropriate to apply the provisional ADI of 0.15mg/kg bw/day or 10mg/day as identified in the joint statement of the ACNFP and COT on >98% pure forms of CBD.
2.11 Allergenicity
62. This CBD isolate comprises >98% pure CBD and the production process for CBD does not introduce any risk of allergenic potential. CBD as a chemical entity, suggests the potential for IgE mediated food allergy is unlikely.
63. The assessment considered whether the remaining 2% of the novel foods composition was likely to be allergenic or elicit food allergic reactions. It was noted that none of the raw materials or processing aids used in the production process are derived from or contain any of the allergenic food ingredients specified under Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Suggesting the potential to elicit reactions in those sensitive to those foods is unlikely.
64. The novel food is unlikely to trigger allergic reactions in the target population under the proposed conditions of use.
3. Discussion
65. The novel food is a CBD isolate ingredient from industrial hemp containing >98% CBD (a Group A CBD ingredient), produced using a multi-step manufacturing process.
66. This CBD isolate is intended to be used as a food ingredient in food supplements, beverages, and confectionary for adults at a defined intake for each product type of up to 10 mg CBD per day; it is not intended to replace any food.
67. In October 2023, the Joint ACNFP and COT subgroup identified a provisional acceptable daily intake (ADI) of 10 mg per day (0.15 mg/kg bw/day) for CBD novel foods containing 98% CBD or above, such as the novel food discussed in this assessment. A weight of evidence approach was used to arrive at a provisional ADI of 10 mg/day (0.15 mg/kg bw/day). The most sensitive human health effects, that this provisional ADI protects against, are seen consistently in the liver and thyroid in a number of studies using >98% pure CBD. This value also takes account of the lack of human-based long-term evidence and evidence regarding potentially vulnerable groups, which is applied here for this CBD isolate (footnote).
68. Based upon the dossier of evidence provided by the applicant, the safety of the novel food was reviewed and evidence to reach a conclusion on safety provided. The evidence presented is consistent with evidence presented to support the development of a provision ADI of 10 mg/day for CBD of 98% purity or above. As such the provisional ADI should be applied to this novel food.
This is subject to the existing advice to consumers that pregnant and breastfeeding women and people taking any prescription medication should avoid the consumption of CBD. Consumers on regular medications should seek advice from a medical professional before using any type of CBD food product. In addition, children and prospective parents trying for a baby are advised against consumption of CBD, as are those who are immunosuppressed, due to remaining data gaps and residual uncertainties concerning the safety of CBD for these groups of consumers. These contraindications would also apply to this novel food.
69. The maximum safe exposure for healthy adults of 70 kg as identified in the provisional ADI is 10mg per day. If the inclusion level of this CBD isolate leads to an intake per individual serving of each product type of 10 mg/day, only one product type per day should be consumed to ensure the provisional ADI is not exceeded. Multiple intakes of products containing CBD on the same day should be avoided to support minimising exposure to below the provisional ADI.
4. Conclusions
70. The ACNFP have undertaken a review of this CBD isolate and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The proposed uses are not considered nutritionally disadvantageous.
71. These conclusions were supported by the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
- annexes to the dossier which relate to the identity of the novel food, the production process, stability, methods of analysis, particle size analysis and toxicology.
- in vitro bacterial reverse mutation test [(ERBC study n.A4291)], in vitro mammalian cell micronucleus test [(ERBC study n.A4292)] and 90-day repeat dose feeding study [(ERBC study n.A4290)].
The members of the ACNFP during the course of the assessment who were; Dr Camilla Alexander White, Dr Anton Alldrick, Dr Kimon Andreas Karatzas, Alison Austin, Professor George Bassel, Dr Mark Berry, Dr Christine Bosch, Professor Dimitris Charalampopoulos, Dr Catharina Edwards, Professor Susan Fairweather-Tait, Professor Paul Frazer, Dr Hamid Ghoddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry J. McArdle, Mrs Rebecca McKenzie, Professor Clare Mills, Dr Antonio Peña-Fernández, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, and Professor Bruce Whitelaw.
72. To note, interests were received from members of the ACNFP, Dr Alldrick declared a potential interest relating to his previous employment and this was considered a potential conflict and as a result he was not present for discussions of CBD by the Committee. Emeritus Prof Harry McArdle declared an interest from his work with EFSA's novel food committee in considering data requirements for CBD. While not seen as a conflict, to avoid Prof McArdle being subject to information that would influence his EFSA work, it was agreed that he would not be present in discussions for CBD by the ACNFP but could supply comments for consideration by the Committee upon review of the minutes.
5. References
Advisory Committee on Novel Foods and Processes (ACNFP) and Committee on Toxicity (COT), 2023. Joint position paper from the Advisory Committee on Novel Foods and Processes (ACNFP) & Committee on Toxicity (COT) on establishing a provisional acceptable daily intake (ADI) for pure form (≥98%) cannabidiol (CBD) in foods, based on new evidence. Published October 2023 DOI: https://doi.org/10.46756/sci.fsa.zcg392
AOAC (2007) Official Methods of Analysis. 18th Edition, Association of Official Analytical chemists, Gaithersburg.
AOAC Cannabis Analytical Science Program (CASP). “AOAC SMPR® 2019.002.” Standard Method Performance Requirements (SMPRs®) for Identification and Quantitation of Selected Residual Solvents in Cannabis-Derived Materials, 9 Oct. 2019
AOAC Official Method 2018.10 Determination of Cannabinoids in Cannabis sativa Dried Flowers and Oils Liquid Chromatography with UV Detection First Action 2018
Audino, Susan et al. “AOAC SMPR® 2017.001. Standard Method Performance Requirements (SMPRs) for Quantitation of Cannabinoids in Cannabis Concentrates.” Journal of AOAC International 100 4 (2017): 1200-1203 .
Borman, Phillip, and David Elder. "Q2(R1) Validation of Analytical PROCEDURES." ICH Quality Guidelines, 2017, pp. 127-166., doi: 10.1002/9781118971147.ch5
https://doi.org/10.1002/9781118971147.ch5
Collins, Arianna C. Extraction Procedures HPLC (AC001). 2020. Midwest Extraction Services Analytical Laboratory, Minnesota
Committee On Mutagenicity Of Chemicals In Food, Consumer Products And The Environment, (MUT/MIN/2020/1), 2020; Committee on Mutagenicity minutes from meeting on 20th February 2020.
Cruijsen, Hans & Poitevin, Eric & Brunelle, Sharon & Almeida, S & Braun, U & Connelly, M & Giuliani, L & Huertas, Raquel & Hui, S & Ikeuchi, Y & Jaudzems, G & Kimura, S & Kittleson, J & Larkin, G & Li, F & McMahon, A & Nagatoshi, M & Piccon, I & Postma, M & Xindong, G. (2019). Determination of Minerals and Trace Elements in Milk, Milk Products, Infant Formula, and Adult Nutrition: Collaborative Study 2011.14 Method Modification. Journal of AOAC INTERNATIONAL. 102. 1845-1863. 10.1093/jaoac/102.6.1845. https://doi.org/10.5740/jaoacint.19-0073
Determination of Chromium, Selenium, and Molybdenum in Infant Formula and Adult Nutritional Products - Inductively Coupled Plasma-Mass Spectrometry: Collaborative Study, Final Action 2011.19. J AOAC Int. 2015 Jun 24. doi: 10.5740/jaoacint.15139. Epub ahead of print. PMID: 26111082. https://doi.org/10.5740/jaoacint.15139
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, Greenwood S, Morrison G and Sommerville K, 2018. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome [GWPCARE1 Part A Study Group]. Neurology, 90, e1204-e1211. https://doi.org/10.1212/WNL.0000000000005254
EC, 2017. Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Commission Implementing Regulation (EU) 2017/2469
EFSA CONTAM Panel (Panel on Contaminants in the Food Chain), 2015. Scientific opinion on the risks for human health related to the presence of Tetrahydrocannabidiol (THC) in milk and other food from animal origin. DOI, 10.2903/j.efsa.2015.4141
EFSA NDA Panel (EFSA Panel on Nutrition and Novel Foods). (2016). Guidance on the Preparation and Presentation of an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA Journal, 14(11), 4594. Https://doi.org/10.2903/j.efsa.2016.4594
ERBC Study No: A4290 [unpublished]. Prepared by European Research Biology Centre, Pomezia RM Italy for Cannaray Brands Ltd., London, United Kingdom. Study Title: Cannabidiol Isolate: 13-week oral toxicity study in rats followed by a 4 week recovery period. Confidential (Test Facility Study No. A4290).
ERBC Study No: A4291 [unpublished]. Prepared by European Research Biology Centre, Pomezia RM Italy for Cannaray Brands Ltd., London, United Kingdom. Study Title: Cannabidiol Isolate: bacterial mutation assay. Confidential (Test Facility Study No. A4291).
ERBC Study No: A4292 [unpublished]. Prepared by European Research Biology Centre, Pomezia RM Italy for Cannaray Brands Ltd., London, United Kingdom. Study Title: Cannabidiol Isolate: in vitro micronucleus test in human lymphocytes. Confidential (Test Facility Study No. A4292).EU (2015) Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 Regulation (EU) 2015/2283 of the European Parliament and of the Council
Grotenhermen, F. (2003) ‘Pharmacokinetics and pharmacodynamics of cannabinoids’, Clinical Pharmacokinetics, 42(4), 327–360. https://doi.org/10.2165/00003088-200342040-00003
Hawksworth, G. and McArdle, K. (2004) ‘Metabolism and pharmacokinetics of cannabinoids’, in Guy, G, Whittle, B and Robson, P (eds.), The Medicinal Uses of Cannabis and Cannabinoids. London: London Pharmaceutical Press. pp. 205–228. Google Scholar
Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol Adverse Effects and Toxicity. Curr Neuropharmacol. 2019;17(10):974-989. doi: 10.2174/1570159X17666190603171901
Izgelov D, Davidson E, Barasch D, Regev A, Domb AJ, Hoffman A. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur J Pharm Biopharm. 2020 Sep;154:108-115. doi: 10.1016/j.ejpb.2020.06.021
Jiang R, Yamaori S, Takeda S, Yamamoto I and Watanabe K, 2011. ‘Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes’, Life Sciences, 89(5–6), 165–170. https://doi.org/10.1016/j.lfs.2011.05.018
Joint position paper from the Advisory Committee on Novel Foods and Processes (ANCFP) & Committee on Toxicity (COT) on establishing a provisional acceptable daily intake (ADI) for pure form (≥98%) cannabidiol (CBD) in foods, based on new evidence; October 2023. https://doi.org/10.46756/sci.fsa.zcg392
Kraemer M, Broecker S, Madea B, Hess C. Decarbonylation: A metabolic pathway of cannabidiol in humans. Drug Test Anal. 2019 Jul;11(7):957-967. doi: 10.1002/dta.2572. Epub 2019 Mar 20. PMID: 30698361. https://doi.org/10.1002/dta.2572
Klotz KA, Grob D, Hirsch M, Metternich B, Schulze-Bonhage A, Jacobs J. Efficacy and Tolerance of Synthetic Cannabidiol for Treatment of Drug Resistant Epilepsy. Front Neurol. 2019 Dec 10;10:1313. DOI: 10.3389/fneur.2019.01313
Lehotay, Steven J. “Supplemental Information for: Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study.” Journal of AOAC INTERNATIONAL, vol. 90, no. 2, 2007. https://doi.org/10.1093/jaoac/90.2.1SUP
Mandrioli, Mara, et al. “Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis Sativa L.” Molecules, vol. 24, no. 11, 2019, p. 2113. https://doi.org/10.3390/molecules24112113
Martin-Santos, R., Crippa, J. A., Batalla, A., Bhattacharyya, S., Atakan, Z., Borgwardt, S., Allen, P., Seal, M., Langohr, K., Farré, M., Zuardi, A. W and McGuire, P. K. (2012). Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Current pharmaceutical design, 18(32), 4966–4979. https://doi.org/10.2174/138161212802884780
‘MDA’, Misuse of Drugs Act 1971, UK Public General Acts1971 c. 38, https://www.legislation.gov.uk/ukpga/1971/38/contents
Mechoulam R, Parker LA and Gallily R, 2002. Cannabidiol: an overview of some pharmacological aspects. Journal of Clinical Pharmacology, 42, 11S-19S. https://doi.org/10.1002/j.1552-4604.2002.tb05998.x
Millar SA, Stone NL, Yates AS and O'Sullivan SE, 2018. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Frontiers in Pharmacology, 9, 1365 [13pp, plus supplementary table]. https://doi.org/10.3389/fphar.2018.01365
OECD (Organisation for Economic Co-operation and Development), 1997. Bacterial reverse mutation test. In OECD guidelines for the testing of chemicals. OECD guideline No 471 (updated & adopted: 21 July 1997). Paris, France: Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264071247-en
OECD (Organisation for Economic Co-operation and Development), 1998. OECD principles of good laboratory practice. Series on principles of good laboratory practice and compliance monitoring, No. 1 (ENV/MC/CHEM(98) 17). Paris, France: Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Chemicals Group and Management Committee. https://doi.org/10.1787/9789264078536-en
OECD (Organisation for Economic Co-operation and Development), 2016. In vitro mammalian cell micronucleus test. In OECD guidelines for the testing of chemicals. OECD guideline No 487 (updated & adopted: 29 July 2016). Paris, France: Organisation for Economic Cooperation and Development (OECD). https://doi.org/10.1787/9789264264861-en
OECD (Organisation for Economic Co-operation and Development), 2018. Repeated dose 90-day oral toxicity study in rodents. In OECD guidelines for the testing of chemicals. OECD guideline No 408 (updated and adopted 27 June 2018). Paris, France: Organisation for Economic Cooperation and Development (OECD). https://doi.org/10.1787/9789264070707-en
Sellers EM, Schoedel K, Bartlett C, Romach M, Russo EB, Stott CG, Wright S, White L, Duncombe P, Chen CF. A Multiple-Dose, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group QT/QTc Study to Evaluate the Electrophysiologic Effects of THC/CBD Spray. Clin Pharmacol Drug Dev. 2013 Jul;2(3):285-94. https://doi.org/10.1002/cpdd.36
Stott CG, White L, Wright S, Wilbraham D, Guy GW. A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol. 2013 May;69(5):1135- 47. https://doi.org/10.1007/s00228-012-1441-0
Stero Biotechs Ltd. Phase 2a Study to Evaluate the Safety, Tolerability and Efficacy of Cannabidiol as a Steroid-sparing Therapy in Steroid-dependent Crohn's Disease Patients. ClinicalTrials.gov Identifier: NCT04056442. Last Update Posted : June 30, 2020. Available online at: A Phase 2a Study to Evaluate the Safety, Tolerability and Efficacy of Cannabidiol as a Steroid-sparing Therapy in Steroid-dependent Crohn's Disease Patients
The Food Supplements (England) Regulations 2003, No. 1387, England; The Food Supplements (England) Regulations 2003
The Food Supplements (Wales) Regulations 2003 (legislation.gov.uk)
The Food Information Regulations 2014, No.1855; The Food Information Regulations 2014
Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A Phase I, Randomized, Double-Blind, Placebo Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs. 2018 Nov;32(11):1053-1067. https://doi.org/10.1007/s40263-018-0578-5
Wheless JW, Dlugos D, Miller I, Oh DA, Parikh N, Phillips S, Renfroe JB, Roberts CM, Saeed I, Sparagana SP, Yu J, Cilio MR; INS011-14-029 Study Investigators. Pharmacokinetics and Tolerability of Multiple Doses of Pharmaceutical-Grade Synthetic Cannabidiol in Pediatric Patients with Treatment-Resistant Epilepsy. CNS Drugs. 2019 Jun;33(6):593-604. doi: 10.1007/s40263-019-00624-4
WHO (World Health Organization), 2018. Cannabidiol (CBD) critical review report. 40th Meeting - Expert Committee on Drug Dependence, Jun. 4-7, 2018, Geneva. Available online: Cannabidiol (CBD) critical review report
Zendulka, O., Dovrtělová, G., Nosková, K., Turjap, M., Šulcová, A., Hanuš, L and Juřica, J. (2016). Cannabinoids and Cytochrome P450 Interactions. Current drug metabolism, 17(3), 206–226. https://doi.org/10.2174/1389200217666151210142051
Abbreviations
| 1H NMR | 1H (proton) nuclear magnetic resonance |
| ACMD | Advisory Council on the Misuse of Drugs |
| ACNFP | Advisory Committee on Novel Foods and Processes |
| ADI | Acceptable Daily Intake |
| ADME | Absorption, Distribution, Metabolism and Excretion |
| AOAC | Association of Official Analytical Chemists |
| ARfD | Acute Reference Dose |
| aw | Water activity |
| bw | body weight |
| CAS | Chemical Abstracts Service |
| CBD | Cannabidiol |
| Cmax | Peak serum concentration |
| COT | Committee on Toxicity |
| CFU | Colony Forming Unit |
| DAD | Diode Array Detector |
| EC | European Commission |
| EFSA | European Food Safety Agency |
| EMA | Environmental Medicines Agency |
| EU | European Union |
| FDA | Food and Drug Administration (USA) |
| FSA | Food Standards Agency |
| FSS | Food Standards Scotland |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GC | Gas chromatography |
| GC-FID | Gas Chromatography- Flame Ionization Detector |
| GLP | Good Laboratory Practice |
| HACCP | Hazards Analysis and Critical Control Points |
| HPLC | High-performance liquid chromatography |
| HS | Headspace |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| IR | Infra-red |
| LOAEL | Lowest Observable Adverse Effect Level |
| LOD | Limit of Detection |
| LOQ | Lomit of Quantification |
| MS | Mass Spectrometry |
| ND | Not Determined |
| NOAEL | No Observable Adverse Effect Level |
| NM | Not measured |
| OECD | Organisation for Economic Co-operation and Development |
| Tmax | Time to maximum concentration |
| USP | United States Pharmacopeia |
| UV | ultra-violet |