ACNFP Safety Assessment: Calcidiol (25-hydroxycholecalciferol monohydrate) as a novel food for use in food supplements
On this page
Skip the menu of subheadings on this page.Reference Number RP35
Regulated Product Dossier Assessment
Assessment finalised: 28th of March 2024
Executive Summary
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in January 2021 from DSM Nutritional Products Ltd (“the applicant”) for the authorisation of Calcidiol, 25-hydroxycholecalciferol monohydrate as a novel food.
The novel food is a new source of vitamin D3 for use as a food supplement targeted at a generally healthy population including pregnant and lactating women, except children under 3 years. It is a vitamer of vitamin D3 (cholecalciferol) and is directly absorbed in the human gut.
To support the FSA and FSS in their evaluation of the application, the Advisory Committee on Novel Foods and Processes (ACNFP) were asked to review the safety dossier and supplementary information provided by the applicant. Please note the Committee did not consider any potential health benefits or claims arising from consuming the food, as the focus of the novel food assessment is to ensure the food is safe, and not putting consumers at a nutritional disadvantage.
The FSA and FSS concluded that the applicant had provided sufficient information to assure the novel food, Calcidiol, was safe under the proposed conditions of use. The anticipated intake levels and the proposed use in food supplements was not considered to be nutritionally disadvantageous.
The views of the ACNFP have been taken into account in the regulatory assessment which represents the opinion of the FSA and FSS.
1. Introduction
1. In January 2021, DSM Nutritional Products Ltd (“the applicant”) submitted a full novel food application for the authorisation of Calcidiol, 25-hydroxycholecalciferol monohydrate, a new source of vitamin D3. The novel food ingredient is a vitamer of vitamin D3 (cholecalciferol) and is directly absorbed by the human gut. Calcidiol is formed from cholestatrienol by chemical synthesis. The novel food contains the monohydrate form of the major circulating metabolite of vitamin D3 and is a source of 1,25-dihydroxyvitamin D3, the biologically active form of vitamin D. Its proposed use is as a food supplement targeted at a generally healthy population including pregnant and lactating women, except children under 3 years.
2. The FSA and FSS have undertaken a safety assessment for Calcidiol under the novel foods legislation, assimilated Regulation (EU) 2015/2283. To support the safety assessment, the ACNFP provided the advice outlined in this opinion to the FSA and FSS.
3. The evaluation by the ACNFP assessed the food safety risks of the novel food and its production, in line with Article 7 of assimilated Commission Implementing Regulation (EU) 2017/2469. The regulatory framework and the technical guidance put in place by the European Food Safety Agency (EFSA) for full novel food applications is retained as the basis and structure for the assessment (EFSA NDA Panel, 2021).
4. Following the review by the ACNFP in June 2021, further information was requested from the applicant concerning the identity of the novel food, the production process, specification, the proposed uses, history of use, ADME and toxicology information on Calcidiol, in order to address information gaps in the initial dossier. The final advice from the Committee was agreed at the 164th meeting, allowing the FSA and FSS to complete the risk assessment.
5. The document outlines the conclusions of the FSA and FSS on the safety of Calcidiol as a novel food.
2. Assessment
2.1 Identity of the novel food
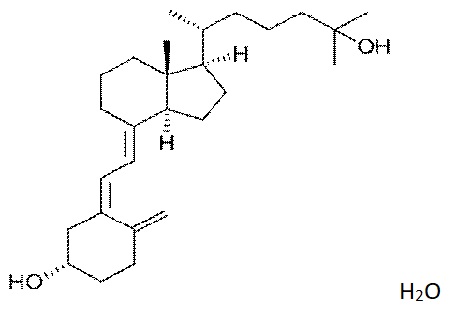
6. The novel food, Calcidiol (Figure 1), 25-hydroxycholecalciferol monohydrate is characterised by the following information:
IUPAC name (3S,5Z,7E)-9,10-secocholesta-5,7-10(19)-triene-3,25-diol monohydrate
Common Synonyms 25-Hydroxycholecalciferol monohydrate; Calcifediol, 25-OH-D3; 25-hydroxyvitamin D3
Synonyms in various reports HyD®, Calcifediol, photoconversion HD3 crystal FG, photoconversion 25-OH-D3 crystal FG
CAS number (Calcifediol
Monohydrate) 63283-36-3
Molecular weight 418.7 g/mol
Molecular formula C27H44O22 .H2O
Figure1. The structural formula of 25-hydroxycholecalciferol monohydrate.
7. The identity of 25-hydroxycholecalciferol monohydrate was demonstrated by infrared absorption spectrometry in comparison with the Ph. Eur. reference standard. The Committee asked questions about the characterisation of the novel food. In response, the applicant explained that nuclear magnetic resonance (NMR) is not the analytical method of choice for characterising 25-hydroxycholecalciferol in the product due to interference from starches used in formulation. To avoid this interference and provide an additional level of detail, high-resolution high performance liquid chromatography-mass spectrometry (HPLC-MS) was used to identify the compound in the product based on comparison of retention times with those of reference material by UV, high-resolution MS and tandem MS (MS/MS). This analysis confirms the identity of the novel food as 25-hydroxycholecalciferol monohydrate.
8. For the purposes of the assessment the term 25-hydroxycholecalciferol monohydrate will be used when referring to the novel food and 25-hydroxycholecalciferol when referring to the form found in aqueous media.
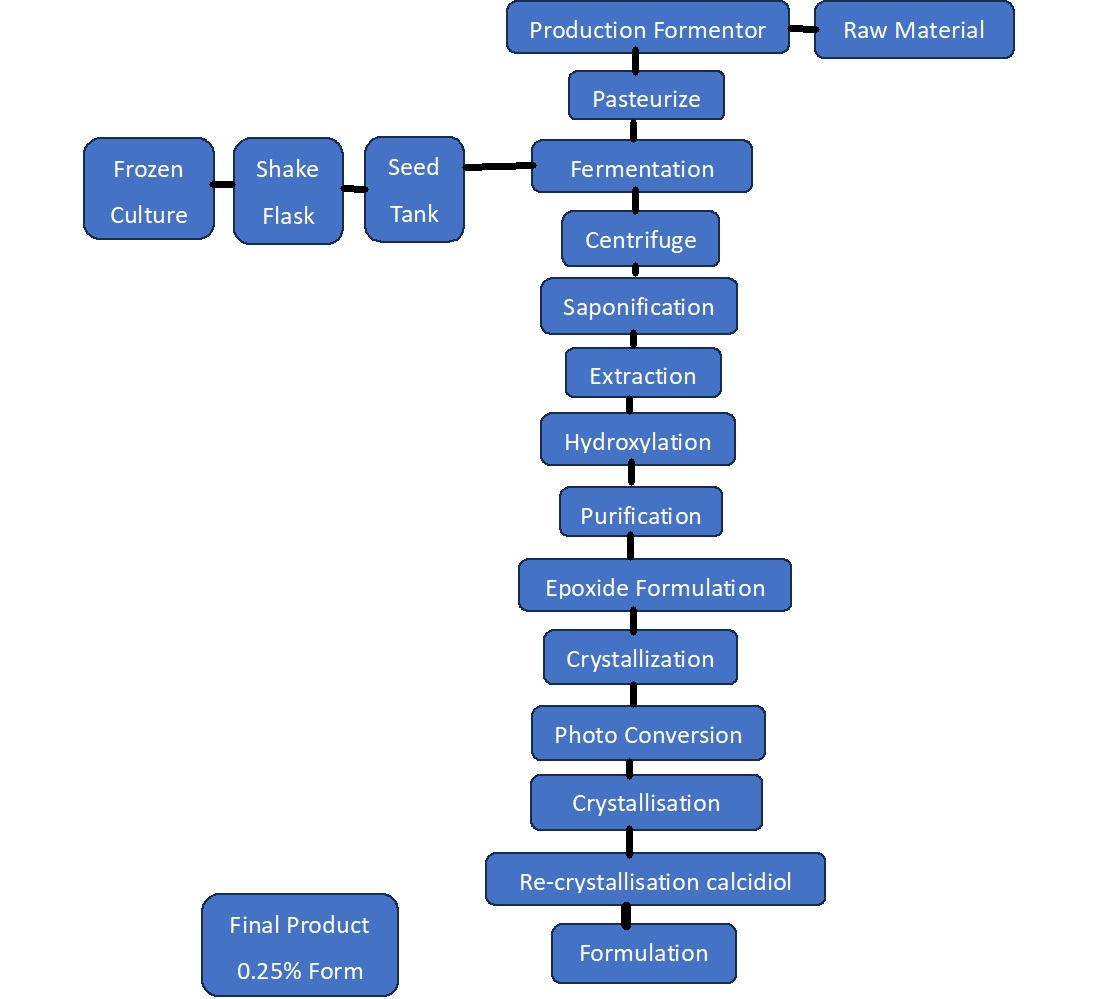
2.2 Production Process
9. 25-Hydroxycholecalciferol monohydrate is manufactured in compliance with the principles of Good Manufacturing Practice (GMP) and Hazard Analysis and Critical Control Points (HACCP).
10. The production process (Figure 2) commences with a yeast fermentation of a genetically engineered strain of Saccharomyces cerevisiae resulting in a mixture of sterols, the main one being trienol.
11. A detailed description of the genetic modification steps leading to the final derivative strain used to produce the novel food was provided. In line with the use of genetically modified microorganisms in the production of other foods the applicant confirmed that no recombinant DNA remains in the final product through analysis performed on three independent batches. As such, it is not subject to the regulatory requirements for genetically modified organisms.
12. Further chemical conversion steps (saponification, extraction, hydroxylation and isomerization by photoconversion) then crystallisation, isolation and drying are used to produce a crude 25-hydroxycholecalciferol monohydrate preparation. After drying, 25-hydroxycholecalciferol monohydrate is recrystalised and a 0.25% w/w diluted product is formulated using starches (permitted as food additives), as excipients, to stabilise the product.
Figure 2. 25-hydroxycholecalciferol monohydrate production flow chart.
13. The Committee concluded that the production process was sufficiently described and did not raise safety concerns.
2.3 Compositional information
14. The applicant undertook compositional analysis to verify the characteristics of the novel food and to confirm the effective management of potential impurities and heavy metals in the novel food (Table 1).
Table 1. Compositional Analysis of the novel food.
|
Parameter |
Lot: UT16090001 |
Lot: UT16090212 |
Lot: UT16090003 |
Lot: UT16090004 |
|
Appearance |
White |
White |
White |
White |
|
Identity |
Corresponds |
Corresponds |
Corresponds |
Corresponds |
|
Assay 25(OH)D3.H2O |
100.6 % |
101.3 % |
101.1 % |
102.2 % |
|
Total related substances |
0.5 % |
0.5 % |
0.6 % |
0.5 % |
|
∆22-25(OH)D3 |
0.2 % |
0.2 % |
0.3 % |
0.2 % |
|
9b,10a-cholesta-5,7-diene-3b,25-diol (25(OH)lumisterol, imp.A) |
0.14 % |
0.14 % |
0.1 % |
0.1 % |
|
Cholesta-5,7-diene- 3b,25-diol (pro25(OH)D3, imp. B) |
0.14 % |
0.14 % |
0.1 % |
0.1 % |
|
(6E)-9,10-secocholesta-5(10),6,8- triene-3b,25-diol (iso25(OH)tachysterol, imp.C) |
0.14 % |
0.14 % |
0.1 % |
0.1 % |
|
(5E,7E)-9,10secocholesta-5,7,10(19)-triene3b,25-diol (transvitamin; imp D). |
0.14 % |
0.14 % |
0.1 % |
0.1 % |
|
Other impurities (each) |
0.09 % |
0.09 % |
0.10 % |
0.10 % |
|
Water content |
4.4 % |
4.4 % |
4.9 % |
4.5 % |
|
Acetone |
840 ppm |
821 ppm |
789 ppm |
840 ppm |
|
Isopropanol |
ND |
ND |
ND |
ND |
|
Heavy metals |
< 10 ppm |
< 10 ppm |
< 10 ppm |
< 10 ppm |
|
As |
< 1.0 ppm |
< 1.0 ppm |
< 1.0 ppm |
< 1.0 ppm |
|
Pb |
< 1.0 ppm |
< 1.0 ppm |
< 1.0 ppm |
< 1.0 ppm |
|
Hg |
< 0.1 ppm |
< 0.1 ppm |
< 0.1 ppm |
< 0.1 ppm |
|
Cd |
< 0.5 ppm |
< 0.5 ppm |
< 0.5 ppm |
< 0.5 ppm |
ND – Not Detected
15. The potential for the bioavailability of 25-hydroxycholecalciferol to be influenced by the presence in the formulation of particles in the nanoparticulate size range was noted. To address this, the applicant undertook testing in line with the European Commission recommendation 2011/69/EU on the testing of nanomaterials. Energy Dispersive X-ray Spectroscopy (TEM-EDX) analysis was performed to determine the geometrical size and elemental composition of particles in the novel food preparation. A 50% threshold was applied. Based on TEM-EDX results, it was concluded that the novel food preparation does not contain nanomaterials.
16. A second test was undertaken to take account of the impact of digestion. The in vitro digestibility of the novel food was tested at concentrations corresponding to 5,10 and 50µg of the novel food. Samples were collected at standard time points and analysed for the novel ingredient by TEM-EDX, Dynamic Light Scattering and LC-MS/MS. The novel ingredient was detected in both soluble and particulate fractions. Number-based size distribution and descriptive parameters of 25-hydroxycholecalciferol in the particulate fraction indicated a median – D50 particle value of 1 µm, confirming that the in vitro digestive process does not induce significant nanostructure formation. The novel food was not, therefore, considered to be a nanomaterial.
17. The data presented indicate the novel food can be consistently produced within the proposed specification.
2.4 Stability
18. The stability of the novel food was assessed. The tests were initially carried out on three batches of the novel food in closed aluminium bottles at 5°C for a period of 12 months. Another three batches of the preparation were tested under a range of conditions including at 15°C for 36 months, 25°C at 60% relative humidity for 36 months and 40°C at 75% relative humidity for a period of 6 months. The food was tested for appearance, identity, assay, water content impurities, appearance, colour, dispersibility, loss on drying and assay. From the evidence of stability during storage for up to 36 months, the proposed shelf life for the 0.25% preparation was estimated to be 36 months when stored at 15°C and 25°C at a relative humidity of 60 ± 5%.
19. The Committee considered the information provided demonstrated the stability of the novel food for 36 months under normal storage conditions.
2.5 Specification
20. The specification parameters for 25-hydroxycholecalciferol monohydrate are presented in Table 2. These are consistent with European and US Pharmacopeia (EP and USP) specifications for 25-hydroxycholecalciferol.
21. The results from four batches of 25-hydroxycholecalciferol monohydrate were provided. It was concluded that the novel food was consistently produced to the proposed specification.
Table 2. Specification for 25-hydroxycholecalciferol monohydrate.
|
Parameter |
Analytical method |
Specification |
|
Appearance |
Visual |
White to almost white crystals |
|
Identity of 25-hydroxycholecalciferol structure compared to reference |
IR, HPLC-UV (254 nm) |
Corresponds |
|
25(OH)D3.H2O |
HPLC-UV (254 nm) |
97.0 % - 103 % |
|
Total related substances |
HPLC-UV (265 nm) |
< 1.5 % |
|
∆22-25(OH)D3 |
HPLC-UV (265 nm) |
< 0.5 % |
|
9β,10α-cholesta-5,7-diene-3β,25-diol |
HPLC-UV (265 nm) |
< 0.5 % |
|
(25(OH)lumisterol, imp. A) |
HPLC-UV (265 nm) |
< 0.5 % |
|
Cholesta-5,7-diene-3β,25-diol (pro-25(OH)D3, imp. B) |
HPLC-UV (265 nm) |
< 0.5 % |
|
(6E)-9,10-secocholesta-5(10),6,8-triene-3β,25-diol (iso-25(OH)tachysterol,imp. C) |
HPLC-UV (265 nm) |
< 0.5 % |
|
(5E,7E)-9,10-secocholesta-5,7,10(19)triene-3β,25-diol (trans-vitamin; imp D). |
HPLC-UV (265 nm) |
< 0.5 % |
|
Other impurities (each) |
HPLC-UV (265 nm) |
< 0.10 % |
|
Water content |
KF (USP 921, method 1c) |
3.8 – 5.0 % |
|
Acetone |
GC Headspace |
< 1000 ppm |
|
Isopropanol |
GC Headspace |
< 500 ppm |
|
Heavy metals |
ICP-MS |
< 10 ppm |
|
As |
ICP-MS |
< 1.0 ppm |
|
Pb |
ICP-MS |
< 1.0 ppm |
|
Hg |
ICP-MS |
< 0.1 ppm |
|
Cd |
ICP-MS |
< 0.5 ppm |
22. While the novel food is the crystalline form of 25-hydroxycholecalciferol monohydrate, the food experienced by consumers will be a preparation containing the novel food. To support the review of safety, analyses of four batches of a 0.25% w/w preparation of 25-hydroxycholecalciferol monohydrate were provided to confirm compliance with the proposed specifications.
23. Microbial analysis of the preparation (Table 3) demonstrated the microbial safety of the novel food in line with General Food Law. The microbiological parameters were within acceptable limits, and it was noted that, given the nature of the novel food and its low water activity, microbial growth was unlikely to be a cause for concern.
Table 3. Microbial Specification 0.25% w/w preparation of 25-hydroxycholecalciferol monohydrate.
|
Microbiological purity: |
|
|
* Total aerobic microbial count |
max. 103 CFU/g |
|
* Total combined yeast/mould count |
max. 102 CFU/g |
|
* Enterobacteria |
< 10 CFU/g |
|
* Escherichia coli |
negative in 10 g |
|
* Salmonella spp. |
negative in 25 g |
|
* Staphylococcus aureus |
negative in 10 g |
|
* Pseudomonas aeruginosa |
negative in 10 g |
24. The Committee considered the information provided on the specifications of the novel food to be sufficient. It did not raise safety concerns.
2.6 History of Use
25. 25-hydroxycholecalciferol monohydrate produced by chemical synthesis has no history of use in the UK or EU and is therefore considered as a novel food.
26. 25-hydroxycholecalciferol is a metabolite of vitamin D3, which is an essential micronutrient for humans and some animals. Vitamin D3 is produced in human skin exposed to the UVB component of sunlight. 25-hydroxycholecalciferol is formed in the liver as a metabolite of vitamin D3, circulates in the blood and is the primary analyte considered when assessing serum vitamin D status. Both vitamin D3 and 25-hydroxycholecalciferol are precursors of the active metabolite, 1,25-dihydroxyvitamin D3 (calcitriol). 25-hydroxycholecalciferol is detectable in meat; however, the substance 25-hydroxycholecalciferol has not previously been extracted or manufactured to be consumed directly as a food.
27. 25-hydroxycholecalciferol monohydrate was introduced in the European Union as a feed additive following approval in Commission Regulation 1443/2006 of its use as vitamin in poultry feed. The authorisation was extended to swine by Commission Regulation 887/2009. There are a number of authorisations across the world, including in the USA for the use of 25-hydroxycholecalciferol monohydrate in chicken and swine feed.
28. There are authorisations in the EU and UK to use 25-hydroxycholecalciferol monohydrate in animal feedstuffs.
2.7 Proposed Use and Anticipated Intake
29. The proposed target population is adults, including pregnant and lactating women, and children from 3 years of age.
30. 25-hydroxycholecalciferol monohydrate will be used as a new source of vitamin D3 in accordance with Directive 2002/46/EC. It is proposed as an alternative to currently authorised forms of vitamin D in some food supplements. The novel food will be marketed as a preparation containing 0.25% w/w 25-hydroxycholecalciferol monohydrate. The applicant proposes a maximum daily intake of 10 µg per day for adults, including pregnant and lactating women, and children above 11 years of age. The proposed maximum daily intake for children 3-10 years is 5 µg per day.
2.8 Absorption, Distribution, Metabolism and Excretion (ADME)
31. 25-hydroxycholecalciferol (also referred to as calcifediol) is the primary circulating metabolite of vitamin D3 (cholecalciferol). The data in this section considers both 25-hydroxycholecalciferol and cholecalciferol because the two are similar and closely interconnected (Van den Berg 1997, Christakos et al. 2010, Borel et al, 2015.
32. The Committee commented that the application did not consider whether use of a metabolite of vitamin D3 might affect the downstream metabolism and homeostatic regulation of circulating vitamin D3 metabolite levels. Vitamin D3 metabolism involves the conversion of cholecalciferol into 25-hydroxycholecalciferol which is then further metabolised. The novel ingredient has the same identity as the metabolite of vitamin D3 from a range of sources and there was no evidence to suggest that they would behave differently in the body.
33. Queries were also raised on the impact on metabolic pathways in the liver of consuming a bolus dose of a vitamer that is highly bioavailable. Data from the applicant did not give reason to believe that 25-hydroxycholecalciferol as a novel food would be metabolised differently from 25-hydroxycholecalciferol coming from other dietary sources or via this pathway, or that it would have wider impacts on feedback regulation, related pathways or vitamin D homeostasis. This is supported by the wider evidence for supplementation with vitamin D3 via the diet.
34. The Committee noted information presented from the literature that suggested potential changes in the metabolism of vitamin D3 by women during pregnancy and lactation. The implications this might have for the safe use of the novel food were explored. Committee members commented that altered metabolism in pregnant women may be linked with an increased risk of vitamin D deficiency for this population. Literature data supplied reported that serum 1,25-dihydroxyvitamin D3 concentrations in response to vitamin D supplementation were similar in pregnant and lactating women and in non-pregnant or non-lactating women (Institute of Medicine, 2011). The proposed maximum dose and conditions of use cover safe consumption by this group.
35. The Committee also highlighted the implications of additional Vitamin D3 exposure with regard to potential toxicity under the proposed conditions of use. Evidence was presented that most individuals in UK and EU populations would not exceed the recommended level for populations i.e. the UK Reference Nutrition Intake (RNI), and the EU Population Reference Intake (PRI) following supplementation with the novel food under the suggested conditions of use.
36. The most recent results from the UK National Diet and Nutrition Survey indicate that, in 2016-2019, mean vitamin D intakes from food sources were below the RNI of 10µg per day in all age groups, at around a fifth to a quarter of the RNI in children and a quarter to a third in adults. When intakes of vitamin D from supplements were taken into account, mean intakes increased to around 29-40% of the RNI for children and 54% for adults 19-64 years, 91% for 65-74 years and 60% for adults aged 75 years and over. There was evidence of low vitamin D status (as indicated by low plasma 25-hydroxycholecalciferol concentrations in blood) in all age groups (Public Health England, 2020).
2.9 Nutritional Information
37. Nutritional data for the novel food itself was not provided as it consists of 25-hydroxycholecalciferol monohydrate. The nutrient content of the food supplement that would be consumed is summarised in Table 4.
Table 4. Nutrient analysis of 25-hydroxycholecalciferol monohydrate-containing preparation.
|
Energy content total |
KJ/100g |
1697 |
|
Energy content total |
Kcal/100g |
400 |
|
Content in: |
|
|
|
Fat |
g/100g |
3 |
|
-of which saturated fatty acids |
g/100g |
3 |
|
-of which mono-unsaturated fatty acids |
g/100g |
0 |
|
-of which poly-unsaturated fatty acids |
g/100g |
0 |
|
-of which trans fatty acids
|
g/100g |
0 |
|
-of which cholesterol |
mg/100g |
0 |
|
Carbohydrate |
g/100g |
90.6 |
|
-of which sugars |
g/100g |
17.5 |
|
-of which polyols |
g/100g |
0 |
|
-of which starch |
g/100g |
73.1 |
|
Fibre |
g/100g |
0 |
|
Protein |
g/100g |
0 |
|
Salt (Sodium content x 2.5) |
g/100g |
1.0 |
38. A range of values has been reported for the bioavailability of 25-hydroxycholecalciferol in humans. The European Food Safety Authority (EFSA) recently published an opinion on the relative bioavailability of 25-hydroxycholecalciferol versus vitamin D3. The body of evidence consisted of 12 randomised controlled trials (RCTs) with low or relatively low risk of bias. In all RCTs the achieved serum 25-hydroxycholecalciferol concentrations per µg/day of vitamin D were higher when subjects consumed 25-hydroxycholecalciferol compared with vitamin D3; however, in the four RCTs that assessed multiple doses mean differences tended to decrease with increasing doses of 25-hydroxycholecalciferol (EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) 2023). The use of 25-hydroxycholecalciferol in food supplements was considered safe at intake levels up to 10 µg/day; the mean relative bioavailability derived in the meta-analysis of all RCTs was 2.4, but it consistently increased with decreasing dose of 25-hydroxycholecalciferol. EFSA concluded that a conversion factor of 2.5 would be appropriate. The Committee agreed and a conversion factor of 2.5 was used in this risk assessment.
39. Based on the data presented, the Committee concluded that 25-hydroxycholecalciferol monohydrate is not expected to be nutritionally disadvantageous and will provide an additional source of vitamin D in the diet.
2.10 Toxicological Information
2.10.1 Genotoxicity
40. The novel food was tested for mutagenicity in a reverse mutation assay using five strains, Escherichia coli WP2 uvrA and Salmonella typhimurium TA1535, TA1537, TA98 and TA100. The test was Good Laboratory Practice (GLP) compliant and in line with OECD Test Guideline (TG) 471. Two independent experiments were conducted using 25-hydroxycholecalciferol monohydrate of 96.9% purity; each was undertaken in the presence and absence of metabolic activation. Concentrations of 3, 10, 33, 100, 333, 1000, 2500 and 5000 μg/plate were used in experiment 1, and concentrations of 10, 33, 100, 333, 1000, 2500 and 5000 μg/plate in experiment 2. No mutagenicity (increase in revertant colony numbers) was observed. 25-hydroxycholecalciferol monohydrate was concluded to be non-mutagenic in this test (Wöhrle and Sokolowski, 2013).
41. A GLP-compliant in vitro mutagenicity test in L5178Y mouse lymphoma cells, with and without metabolic activation, was conducted in line with OECD TG 490. The batch tested was 25-hydroxycholecalciferol monohydrate of purity 96.9%. In the first experiment, 25-hydroxycholecalciferol monohydrate was tested up to concentrations of 7.5 and 25 μg/ml in the presence and absence of metabolic activation (S9 mix) for 3 hours. In the second experiment, 25-hydroxycholecalciferol monohydrate was tested for 24 h up to concentrations of 5 μg/ml in the absence of S9 mix. 25-hydroxycholecalciferol monohydrate was not mutagenic in the mouse lymphoma L5178Y test system (Remus and Verspeek-Rip, unpublished report, 2016).
42. An in vivo micronucleus test was undertaken in rat bone marrow at doses up to 1000 mg/kg-bw. The test was GLP-compliant and in line with OECD TG 474. The test item was 25-hydroxycholecalciferol monohydrate at 96.9% purity. Changes in haemotological parameters indicated exposure to the novel food or its metabolites in the target tissue. The study showed no increase in micronucleated erythrocytes in treated animals, indicating that the test substance was not genotoxic in this test. (Verban et al. - unpublished report, 2016).
43. A GLP-compliant in vitro chromosome aberration test using human lymphocytes was conducted according to OECD TG 473 (Weber and Schulz, 2005). Two experiments were undertaken. The highest applied concentration was 100 μg/mL of the test item, approximately 0.2 mM. In neither experiment was a statistically significant or biologically relevant increase in the number of cells carrying structural chromosomal aberrations observed, either in the absence or presence of metabolic activation. 25-hydroxycholecalciferol was therefore concluded not to be clastogenic (Weber E & Schulz M, unpublished report, 2005).
2.10.2 Sub-chronic toxicity
44. The design of the 90-day study submitted in support of this application (Thiel et al, 2014c) was informed by results presented in a primary research publication (Shepard and DeLuca, 1980) and a 90-day study on vitamin D3 (Thiel et al, 2007).
45. Shepard and DeLuca (1980) conducted a 14-day study in which male albino rats were treated orally with vitamin D3 or 25-hydroxycholecalciferol in Wesson oil at total doses increasing by tenfold intervals from 0.65 – 6,500 nmol (Vitamin D3) and 0.46 – 4,600 nmol (25-hydroxycholecalciferol) per day. There was no vehicle control group. The doses were not corrected for body weight; however, based on the anhydrous molecular weights of vitamin D3 and 25-hydroxyvitamin D and the average body weight of rats on receipt at the test facility the maximum daily doses administered were approximately 2.3 – 22,750 μg/kg bw/day for vitamin D3 and 1.7 – 16,770 μg/kg bw/day for 25-hydroxycholecalciferol.
46. “Signs of excessive intake of vitamin D3” were observed at the top two doses of vitamin D3 and the top dose of 25-hydroxycholecalciferol (equivalent to approximately 2275 and 22,750 μg /kg bw/day for vitamin D3 and 16,770 for 25-hydroxycholecalciferol). Death occurred in 9/10 rats treated with vitamin D3 at 22,750 μg/kg bw/day. Observations at 2275 μg /kg bw/day vitamin D3 and 16,770 μg/kg bw/day 25-hydroxycholecalciferol included reduced plasma phosphorus concentrations, hypercalcaemia and greyish-white mottling of the kidneys, which was considered to be consistent with calcification. No histopathological analysis was undertaken.
47. A GLP and OECD TG 408-compliant 90-day study of vitamin D3 was subsequently undertaken in male and female Crl:WI rats (Thiel et al, 2007). Satellite groups (control and top dose only) were allowed 28 days’ recovery after cessation of dosing. The substance tested contained vitamin D3 in a powdered formulation which included a number of excipients (ethoxyquin, edible fat, plant protein, maltodextrin, silicon dioxide and water). Some of the excipients, which included antioxidants, may have been biologically active.
48. The vitamin D3 formulation was administered via the diet at nominal doses of 0, 0.5, 1.5, 4.4 and 13.2 mg/kg bw/day (equivalent to 0, 7, 20, 60 and 180 μg/kg bw/day of the active ingredient) based on group mean body weights and food consumption measured in week 3 onwards and adjusted weekly. These doses corresponded approximately to the lower mid-range of those reported by Shepard and DeLuca (1980). Hyperostosis was observed in the femur (6/10 males and 6/10 females) and sternum (9/10 males and 10/10 females) at the highest dose administered (equivalent to 180 μg/kg bw/day active ingredient). Nephrotoxicity was also observed at the top dose, with evidence of mineralisation in the kidney and other organs. The nephrotoxicity and renal mineralisation were not reversed in top dose animals at the end of the recovery period.
49. No histopathological findings were reported at doses up to 4.4 mg/kg bw/day of test substance (60 μg/kg bw/day of active ingredient) and the clinical chemistry findings at these doses (including hypercalcaemia) were therefore considered non-adverse. The study authors therefore identified a No Observed Adverse Effect Level (NOAEL) of 4.4 mg/kg bw/day of test substance from this study. The proposed NOAEL for the active ingredient, vitamin D3, from this study was, therefore, 60 μg /kg bw/day.
50. The 90-day study submitted in support of the application under review (Thiel et al, 2014c) was also OECD TG 408 and GLP-compliant. It was conducted in Crl:WI (Han) rats and the doses used were similar to those used in the vitamin D3 study (Thiel et al, 2007). The substance tested contained 25-hydroxycholecalciferol (1.34% w/w) in a powdered formulation containing a number of excipients (dl-alpha-tocopherol, coconut oil, modified food starch, maltodextrin, sodium ascorbate and silicon dioxide). Some of the excipients, which included antioxidants, may have been biologically active; given the lack of an excipient control in this study it is impossible to exclude this possibility.
51. The 25-hydroxycholecalciferol formulation was administered via the diet at nominal doses of 0, 0.5, 1.5, 4.5 and 13.4 mg/kg bw/day (equivalent to 0, 7, 20, 60 and 180 μg/kg bw/day of the active ingredient) based on group mean body weights and food consumption measured in week 3 onwards and adjusted weekly. Chemical analyses of dietary preparations were conducted during Weeks 1, 8 and 13 of the study to assess accuracy, homogeneity and stability over 14 days.
52. Analysis of plasma levels of 25-hydroxycholecalciferol indicated that animals in the treatment groups were adequately exposed to the active ingredient. Plasma levels of 25-hydroxycholecalciferol rose upon treatment and peaked after 4 weeks of treatment at all doses tested. Upon cessation of treatment, 25-hydroxycholecalciferol levels in plasma returned to basal levels.
53. Treatment-related mineralisation of the renal pelvis was observed in both sexes at doses of 1.5 mg/kg bw/day of test substance and above (20 μg/kg bw/day of active ingredient) and in females at the lowest dose tested (0.5 mg/kg bw/day of test substance, equivalent to 7 μg/kg bw/day of active ingredient). At the top dose this persisted to the end of the recovery period; it was, however, not considered adverse by the study authors because of the absence of clinical chemistry findings suggesting kidney dysfunction. The NOAEL suggested by the study authors was, therefore, 13.4 mg/kg bw/day of their test substance, equivalent to 180 μg/kg bw /day of the active ingredient, 25-hydroxycholecalciferol.
54. A separate commentary on the histopathological findings from this study was provided by the applicant (Hard, 2014; note that this is not an independent opinion because Hard was a coauthor of the study under review). The commentary states that mineralisation of the renal pelvis is more common in rats than in any other species of laboratory animal and that the mineralisation observed by Thiel et al (2014c) is distal to the sites of resorption and excretion. According to Hard, therefore, it would not be expected to affect renal function. Furthermore, the commentary states, the pattern of mineralisation observed is not consistent with that of hypercalcaemia due to excess vitamin D3. It is, rather, attributed by Hard to the hygroscopic nature of the test substance. This may have been due to the nature of the excipients used in the study, whose main objective was to provide reassurance regarding the use of 25-hydroxycholecalciferol in animal feedstuffs. Such excipient effects cannot be excluded because of the absence of an excipient control in this study.
55. The Committee concluded that the mineralisation observed indicates disruption of kidney function at all doses in female rats and in all but the lowest dose in males. It is not, therefore, possible to derive a NOAEL from this study. If the lowest dose used, 7 μg/kg bw/day, which caused renal mineralisation in female rats only, is taken as a Lowest Observed Adverse Effect Level (LOAEL) this yields a margin of safety of 49 for a 70 kg adult ingesting 25-hydroxycholecalciferol at a dose of 10 mg/day or 21 for a 15 kg child taking 5 mg/day. Given the human safety data provided, this provides sufficient reassurance for the use of 25-hydroxycholecalciferol at the proposed doses in humans.
2.10.2 Human Studies
56. Several trials have compared the effectiveness of vitamin D3 and 25-hydroxycholecalciferol supplementation in healthy volunteers and vulnerable populations.
57. In humans, excess ingestion of vitamin D3 is associated with pain, conjunctivitis, anorexia, fever, chills, thirst, vomiting, and weight loss. These symptoms are associated with hypercalcemia and are the core marker for vitamin D toxicity. No intoxication as measured by hypercalcemia has been reported in humans at serum 25-hydroxycholecalciferol levels below 500 nmol/L (Heaney, 2008, and Hathcock et al., 2007). No signs of hypercalcemia were observed in human trials; indeed, serum calcium levels did not exceed the reference range, nor did 25-hydroxycholecalciferol levels in serum exceed those of control groups.
58. Data were also supplied on the use of vitamin D by pregnant women in human trials. It was noted that a number of trials had been undertaken in pregnant women and meta-analysis had been performed. (Perez-Lopez et al., 2015; De-Regil et al., 2016; Yang et al., 2015). Most involved supplementation after 12 weeks gestation, when organogenesis was complete.
59. Few studies have been conducted early in pregnancy to address specific medical conditions such as hypoparathyroidism resulting in hypocalcaemia. One study reported that pregnant women with hypoparathyroidism received up to 5000 µg/d of vitamin D without adverse effects attributable to supplementation on the infants (Roth DE, 2011).
2.10.4 Conclusions on toxicological data
60. It was not possible to derive a NOAEL from the study of Thiel et al, 2014; however, if the lowest dose used, 7 μg/kg bw/day, which caused renal mineralisation in female rats only, is taken as a LOAEL this yields a margin of safety of 49 for a 70 kg adult ingesting 25-hydroxycholecalciferol at a dose of 10 mg/day or 21 for a 15 kg child taking 5 mg/day. Given the human safety data provided, this provides sufficient reassurance for the use of 25-hydroxycholecalciferol at the proposed doses in humans.
61. To further support the demonstration of the safety of the novel food ingredient an approach comparing the proposed intake to the tolerable upper intake level was adopted.
62. The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) defined an adequate intake (AI) of 15 µg per day for healthy individuals over one year of age (EFSA 2016), while the Upper Level of vitamin D3 for adults identified by EFSA and the Institute of Medicine (IOM) is 100 µg/day. Assuming that 25-hydroxycholecalciferol is 2.5 times more bioavailable than vitamin D3, the adjusted upper intake would be 40 µg/day for adults. This is greater than the proposed dose (10 µg/day) and lower than levels tolerated in human clinical studies in adults including elderly and unhealthy subjects, in which up to 50 µg/d were safely ingested for up to 4 weeks (Barger-Lux et al., 1998) and 20 µg/d could be safely administered for up to 6 months (e.g. Kunz et al. 2016).
63. The Committee sought further information on the rationale for the proposed use levels for children aged 3-10 years. A range of bioavailability values was presented, suggesting additional sources of uncertainty that were yet to be characterised.
64. The potential exposure from the novel food at the proposed use level and vitamin D from other sources was calculated to be 49.4 μg/day for the 3-10 years age group. This level is close to the tolerable upper intake (UL) identified for vitamin D from all oral consumption routes by EFSA for this age group of 50µg per day (EFSA 2023). The ACNFP highlighted that risk managers may wish to consider whether consumers would benefit from further information to minimise the potential for foreseeable misuse in this age group where exposure is close to the tolerable upper intake. The Committee considered the margin of safety between the proposed intake and the UL was appropriate. As such the maximum intake level of the novel food of 5μg/day is considered safe for children aged 3 – 10 years.
2.11 Allergenicity
65. It was noted that the protein content of the novel food is very low. The Committee considered allergic reactions to residual protein in the 25-hydroxycholecalciferol monohydrate ingredient to be unlikely.
3. Discussion
66. The novel food considered in this application is Calcidiol, 25-hydroxycholecalciferol monohydrate, which is a new source of vitamin D3 intended as a replacement for other vitamin D ingredients in supplements. The novel food ingredient is a vitamer of vitamin D3 (cholecalciferol). It is formed from cholestatrienol by chemical synthesis and is directly absorbed by the human intestine. The proposed use is as a food supplement targeted at a generally healthy population including pregnant and lactating women and children from 3 years of age.
67. The identity of the novel food was confirmed. No specific issues were identified in the assessment of the production.
68. The applicant proposes a maximum daily intake of 10 µg per day for adults, including pregnant and lactating women, and children aged 11 years and over. It was not possible to derive a NOAEL from the study of Thiel et al, 2014; however, if the lowest dose used, 7 μg/kg bw/day, which caused renal mineralisation in female rats only, is taken as a LOAEL this yields a margin of safety of 49 for a 70 kg adult ingesting 25-hydroxycholecalciferol at a dose of 10 µg/day or 21 for a 15 kg child taking 5 µg/day. Given the human safety data provided, this provides sufficient reassurance for the use of 25-hydroxycholecalciferol at the proposed doses in humans.
69. Taking into account the 2.5-fold difference in bioavailability between vitamin D3 and 25-hydroxycholecalciferol, the Committee calculated that the tolerable upper intake level for 25-hydroxycholecalciferol monohydrate in adults would be 40 µg/day based on the value of 100 µg/day for vitamin D3. This is consistent with the results from human trials which suggest that the level of the novel food associated with adverse effects such as hypercalcemia is much higher. EFSA, in 2021, concluded: “Regarding achieved serum 25(OH)D, the studies providing supplemental calcidiol intake of 10 or 5 µg/day in various adult populations did not raise mean 25(OH)D concentrations above 107 and 52.2 nmol/L respectively. These concentrations exceed the serum 25(OH)D of 50 nmol/L which was considered as the ‘suitable target value’ by the Panel when setting an adequate intake (AI) for vitamin D (EFSA NDA Panel, 2016b), but are in the range of concentrations (i.e. below 200 nmol/L) unlikely to pose a risk of adverse health outcomes such as hypercalciuria, hypercalcaemia or nephrocalcinosis” (EFSA NDA Panel 2012, 2016b, 2018).
70. The applicant proposes a maximum daily intake of 5 µg per day for children aged 3-10 years. This is based on ensuring that total exposure to vitamin D and its metabolites do not exceed the UL identified for vitamin D from all oral consumption routes by EFSA for this age group (50µg per day). It is noted that the proposed estimated exposure from all sources is for the 95th percentile of consumers in this age group of 49.4μg/day, which is close to the upper tolerable intake when the novel food is used in a preparation containing 0.25%w/w 25-hydroxycholecalciferol monohydrate.
71. The Committee notes that evidence from RCTs, as reported by EFSA (2023), indicates that vitamin D supplementation at doses up to 179 μg/day for 3–12 months does not increase the risk of persistent hypercalcaemia or hypercalciuria in children and adolescents aged 5–18 years. The dose administered is equivalent to approximately 70 µg/day of 25-hydroxycholecalciferol monohydrate, using the conversion factor of 2.5 (EFSA 2023.
72. In considering the safety of the novel food, total exposure to vitamin D3 from the novel food and other sources was explored. The expectation was that consumption of 25-hydroxycholecalciferol monohydrate would replace existing consumption of vitamin D food supplements. Reassurance was sought on how, given the higher bioavailability suggested for 25-hydroxycholecalciferol monohydrate, increased overall exposure to vitamin D3 metabolites would be prevented. It was noted that the product would be sold as an ingredient as a 0.25% preparation and the maximum levels proposed were set to provide an acceptable intake. On this basis the ACNFP suggested that risk managers may wish to consider whether information for consumers would be beneficial to minimise the potential of consuming multiple products containing vitamin D3 or 25 -hydroxycholecalciferol monohydrate on the same day.
73. The Committee also noted comments by the EFSA Panel on Nutrition, Novel Foods and Food Allergens regarding uncertainties in calculated combined exposures to vitamin D in the general population, given the fact that the range of foods fortified with vitamin D as well as food supplements containing a high dose of vitamin D has increased in recent years. The EFSA Panel noted that, depending on the latitude and the time of the year endogenous cutaneous vitamin D synthesis creates additional uncertainty and may affect serum 25-hydroxycholecalciferol concentrations.
4. Conclusion
74. The FSA and FSS have undertaken the assessment of 25-hydroxycholecalciferol monohydrate and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake levels and the proposed use was not considered to be nutritionally disadvantageous.
75. These conclusions were based on the information in the novel food dossier submitted by the applicant plus the supplementary information.
Acknowledgements
With thanks to the members of the ACNFP during the course of the assessment who were; Dr Camilla Alexander White, Dr Anton Alldrick, Alison Austin, Dr Mark Berry, Professor Dimitris Charalampopoulos, Professor Susan Fairweather-Tait, Professor Paul Fraser, Dr Hamid Ghoddusi, Dr Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry McArdle, Rebecca McKenzie, Professor Clare Mills, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, Professor Bruce Whitelaw, Dr Cathrina Edwards, Professor George Bassel, Dr Kimon-Andreas Karatzas, Dr Christine Bosch and Dr Antonio Peña-Fernández.
References
Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF (1998) Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int 8(3):222-230. https://doi.org/10.1007/s001980050058
Borel P, Caillaud D, Cano NJ (2015) Vitamin D Bioavailability: State of the Art. Crit Rev Food Sci Nutr 55(9):1193-1205. https://doi.org/10.1080/10408398.2012.688897
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (2016) Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev 96:365-408. https://doi.org/10.1152/physrev.00014.2015
Commission Regulation (EC) No 1443/2006 of 29 September 2006 concerning the permanent authorisations of certain additives in feedingstuffs and an authorisation for 10 years for a coccidiostat. Queen's Printer of Acts of Parliament. Available at: https://www.legislation.gov.uk/eur/2006/1443.
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Queen's Printer of Acts of Parliament. Available at: https://www.legislation.gov.uk/eur/2008/1333/contents.
Commission Regulation (EC) No 887/2009 of 25September 2009 concerning the authorisation of a stabilised form of 25-hydroxycholecalciferol as a feed additive for chickens for fattening, turkeys for fattening, other poultry and pigs. Queen's Printer of Acts of Parliament. Available at: https://www.legislation.gov.uk/eur/2009/887/contents
De-Regil LM, Palacios C, Lombardo LK, Rena-Rosas JP (2016) Vitamin D supplementation for women during pregnancy (Review). The Cochrane Database Of Systematic Reviews (1), CD008873. doi:10.1002/14651858.CD008873.pub3.
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2016). Dietary reference values for vitamin D. EFSA Journal, 14(10), p.e04547. doi: https://doi.org/10.2903/j.efsa.2016.4547.
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2021). Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283 (Revision 1). Published 26 March. https://doi.org/10.2903/j.efsa.2011.2170
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) (2021). Scientific Opinion on Safety of calcidiol monohydrate produced by chemical synthesis as a novel food. EFSA J 19 (7). https://doi.org/10.2903/j.efsa.2021.6660
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) (2023). Scientific opinion on the tolerable upper intake level for vitamin D, including the derivation of a conversion factor for calcidiol monohydrate. EFSA J. 2023 Aug 8;21(8):e08145.
https://doi.org/10.2903/j.efsa.2023.8145
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) (2024). Scientific opinion on the tolerable upper intake level for vitamin D, including the derivation of a conversion factor for calcidiol monohydrate. https://doi.org/10.2903/j.efsa.2023.8145
Hard, G.C. (2014) Expert Review Of Histological Changes In Rat Kidney from a 90-Day Toxicity Study with Orally Administered DSM047117. Prepared for: DSM Nutritional Products AG, Wurmisweg 576, CH-4303 Kaiseraugst, Switzerland. Submitted as an appendix to Thiel et al, 2014c. DSM proprietary unpublished study.
Hathcock JN, Shao A, Vieth R, Heaney R (2007) Risk Assessment for vitamin D. Am J Clin Nutr 85:6-18. https://doi.org/10.1093/ajcn/85.1.6
Heaney RP (2008) Vitamin D: criteria for safety and efficacy. Nutrition Reviews 66(Suppl 2): S178-S181. https://doi.org/10.1111/j.1753-4887.2008.00102.x
Institute Of Medicine (2011) Dietary Reference Intakes for Calcium and vitamin D. ISBN 978-0-309-16394-1, available at http://www.nap.edu/catalog.php?record_id=13050.
Iris Kunz, Mareike Beck, Rotraut Schoop and Stephane Etheve (2016). Response of serum 25-hydroxyvitamin D to different doses of Calcifediol 0.25 SD/S compared to vitamin D3 supplementation: A randomized, controlled, double blind, long term pharmacokinetic study. DSM proprietary unpublished study.
Perez-Lopez FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, Hernandez AV (2015) Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 103(5):1278-1288e. https://doi.org/10.1016/j.fertnstert.2015.02.019
Public Health England. (2020). NDNS: results from years 9 to 11 (combined) – statistical summary. GOV.UK. https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019/ndns-results-from-years-9-to-11-combined-statistical-summary
Remus T and Verspeek-Rip C, 2016. Evaluation of the mutagenic activity of DSM047117 in an in vitro mammalian cell gene mutation test with L5178Y mouse lymphoma cells (Study conducted at WIL Research Europe B.V., 5231 DD's Hertogenbosch, The Netherlands; WIL study number 511352). DSM Proprietary unpublished data.
Roth DE (2011) Vitamin D supplementation during pregnancy: safety considerations in the design and interpretation of clinical trials. J Perinatology 31:449-459.
https://doi.org/10.1038/jp.2010.203
Shepard RM and DeLuca HF (1980) Plasma concentration of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch Biochem Biophys 202(1):43-53. https://doi.org/10.1016/0003-9861(80)90404-X
Thiel A, Scase K, Hofman P, Kohnlein M and Martland MF, (2007). 3-month oral toxicity with Rovimix D3-500 by dietary administration followed by a 4-week recovery period in Winstar rats. DSM proprietary unpublished data.
Thiel A, Van Otterdijk F, Etheve S, Schierle J and Hard G, (2014). 90-day oral Toxicity study with DSM047117 by Dietary administration in the rat followed by a 28-day recovery period (Study performed at: WIL Research Europe B.V., 5231 DD s. Hertogenbosch, The Netherlands, WIL Study Number: Project 503200). DSM proprietary unpublished data.
Turck, D. et al. (2021) “Safety of calcidiol monohydrate produced by chemical synthesis as a novel food pursuant to regulation (EU) 2015/2283,” EFSA Journal, 19(7). Available at: https://doi.org/10.2903/j.efsa.2021.6660.
Van den Berg H (1997) Bioavailability of vitamin D, Eur J Clin Nutr 51 (Suppl 1), S76-S79.
https://doi.org/10.1038/sj.ejcn.1600384
Verbaan IAJ and Remus T, 2016. DSM047117: Micronucleus test in bone marrow cells of the rat. DSM Proprietary unpublished data.
Weber E and Schulz M, 2005. Chromosome Aberration Test in Human Lymphocytes in vitro with Calcifediol. DSM Proprietary unpublished data.
Wöhrle T and Sokolowski A, 2013. DSM047117: Salmonella typhimurium and Escherichia coli reverse mutation assay. (Study conducted at Harlan CCR; D-64380 Rossdorf; Harlan CCR study Number 1533500). DSM Proprietary unpublished data.
Yang N, Wang L, Li Z, Chen S, Li N, Ye R (2015) Effects of vitamin D supplementation during pregnancy on neonatal vitamin D and calcium concentrations: a systematic review and meta-analysis. Nutrition Research 35:547-556. https://doi.org/10.1016/j.nutres.2015.04.010