ACNFP Advice on the safety of lacto-N-fucopentaose I (LNFP-l) and 2'-fucosyllactose (2'-FL) as a novel food
On this page
Skip the menu of subheadings on this page.Reference Number RP549
Food Standards Agency (FSA) and Food Standards Scotland (FSS)
Regulated Product Dossier Assessment
Assessment finalised: 26th of April 2023
Summary
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in March 2021 from Glycom A/S, Denmark (“the applicant”) for the authorisation of a mixture of lacto-N-fucopentaose I (LNFP-l) and 2'-fucosyllactose (2'-FL) as a novel food.
The novel food is a mixture of LNFP-l and 2'-FL which is intended to be used as a source of human identical milk oligosaccharides. LNFP-l/2'-FL is manufactured by microbial fermentation using a genetically modified strain of Escherichia coli K-12, and then refined to yield the purified novel food.
This new application is seeking to use the novel food within the following food categories: dairy products and analogues, bakery wares, foods for special groups, beverages, and also as a food supplement. Food supplements are not intended to be used if other foods with added LNFP-l/2’-FL or breast milk are consumed the same day.
To support the FSA and FSS in their evaluation of the application, the Advisory Committee on Novel Foods and Processes (ACNFP) were asked to review the safety dossier and supplementary information provided by the applicant. The Committee concluded that the applicant had provided sufficient information to assure the novel food, LNFP-l/2’-FL, was safe under the proposed conditions of use. The anticipated intake levels and the proposed use in foods and food supplements was not considered to be nutritionally disadvantageous and does not mislead consumers.
1. Introduction
1. To support the risk assessment, the ACNFP provided the advice outlined in this opinion to the FSA and FSS.
2. Following the review by the ACNFP at their meeting in June 2022, further clarification was requested concerning the production process of LNFP-l/2'-FL, in order to address information gaps in the dossier. The assessment was completed at the 157th meeting, following the review of the information provided.
3. This document outlines the conclusions of the ACNFP on the safety of LNFP-l/2'-FL as a novel food.
2. Assessment
2.1 Identity of novel food
4. The novel food is a mixture of oligosaccharides containing lacto-N-fucopentaose-l (LNFP-l) and 2'-fucosyllactose (2'-FL), which is a purified white to off-white powder or agglomerate. The content of the novel food is ≥ 75.0% LNFP-l/2'-FL by dry weight, where ≥ 50% is LNFP-l (dry weight) and ≥ 15% is 2’-FL (dry weight). The total saccharide content of the novel food is ≥ 90% dry weight due to the presence of other saccharides present in smaller quantities.
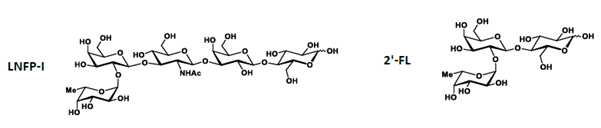
5. LNFP-l is a pentasaccharide consisting of L-fucose, 2 molecules of D-galactose, N-acetylglucosamine, and D-glucose (see Diagram 1). LNFP-1 is characterised by the chemical formula: C32H55NO25; molecular mass: 853.77 g/mol; CAS number: 7578-25-8; CAS name: O-6-Deoxy-α-L-galactopyranosyl-(1→2)-O-β-D-galactopyranosyl-(1→3)-O-2-(acetylamino)-2-deoxy-β-D-glucopyranosyl-(1→3)-O-β-D-galactopyranosyl-(1→4)-D-glucose.
6. 2’-FL is a trisaccharide consisting of L-fucose, D-galactose, and D-glucose (see Diagram 1). 2’-FL is characterised by the chemical formula: C18H32O15; molecular mass: 488.44 g/mol; CAS number: 41263-94-9; CAS name: O-6-Deoxy-α-L-galactopyranosyl-(1→2)-O-β-D-galactopyranosyl-(1→4)-D-glucose.
Diagram 1: The molecular structures of LNFP-l and 2’-FL.
7. The structures of LNFP-l and 2'-FL have been confirmed by liquid chromatography – mass spectrometry (LC-MS) and liquid chromatography – tandem mass spectrometry (LC-MS/MS). Further confirmation on the structure of LNFP-l was provided by nuclear magnetic resonance (NMR) spectroscopy: 1H-NMR, 13C-NMR and 2D-NMR studies, and compared with published data. 2D-NMR studies including double-quantum filtered 1H-1H-correlation spectroscopy, total correlation spectroscopy, Nuclear Overhauser Effect spectroscopy, phase-sensitive 1H-13C-heteronuclear single quantum correlation and phase-sensitive 1H-13C-heteronuclear multiple bond correlation provided unequivocal evidence on the structure of LNFP-l.
8. Information to support this characterisation was provided for six batches of the novel food. Confirmation that the LNFP-l and 2'-FL in the novel food are equivalent to LNFP-l and 2'-FL found in human breast milk was provided by comparative 1H-NMR and 2D NMR analysis.
2.2 Production Process
9. The production microorganism used to manufacture the novel food is a genetically modified derivative of E. coli K-12 DH1 MDO that functions as a processing aid as defined in Article 3(2)(b) of Regulation (EC) No.1333/2008 on food additives, as retained in UK law. A novel food produced by a GMO does not fall under the remit of the GMO legislation, Regulation (EC) No 1829/2003, as retained in UK law, or Regulation (EC) No 1830/2003, as retained in UK law, when the production microorganism, E. coli K-12, is removed during the manufacturing process and therefore no recombinant DNA remains. This has been confirmed in the compositional analysis as detailed below.
10. The novel food is classified as category 1 under the EFSA GMO guidance: chemically defined purified compounds and their mixtures in which both genetically modified microorganisms (GMMs) and newly introduced genes have been removed, under EFSA guidance, which categorises GMMs and their products for risk assessment purposes (EFSA GMO Panel, 2011), which the FSA have retained for the purposes of technical review.
11. The microorganism, E. coli K-12, has not been adopted into the Qualified Presumption of Safety list; however, other human identical milk oligosaccharides manufactured using the same strain of microorganism are present in the List of Novel Foods, Regulation (EU) 2017/2470, as retained in UK law. This includes 2'-FL, LNnT (Lacto-N-neotetraose), 2'-FL/DFL (difucosyllactose), LNT (Lacto-N-tetraose), 3'-SL (3’-sialyllactose sodium salt) and 6'-SL (6’-sialyllactose sodium salt).
12. Information on the hazard identification, hazard characterisation, and exposure assessment for the genetically modified derivative of E. coli K-12 DH1 MDO was provided in line with EFSA guidance (EFSA GMO Panel, 2011).
Diagram 2: Overview of the Manufacturing Process for LNFP-l and 2'-FL.
|
Stage 1 |
Upstream Processing (USP) |
|
Steps |
1 Media Preparation 2 Propagation 3 Seed Fermentation 4 Fermentation Phases 5 Removal of microorganism* |
|
Stage 2 |
Downstream Processing (DSP) |
|
Steps |
6 Purification/Concentration 1* 7 Ion Removal 8 Decolourisation* 9 Purification/Concentration 2* 10 Drying 11 Sampling and Packaging 12 Quality Control & Batch Release |
* After the marked steps, additional sterile filtration (microfiltration) is performed to maintain low microbial load during all times of downstream processing and to ensure high microbial quality of the final ingredient. These steps are further reassurance of absence of the production microorganism in final ingredient.
13. Stage 1 of the production process involves the conversion of D-lactose and D-glucose (D-sucrose or glycerol are alternatives to D-glucose) to LNFP-l and 2'-FL by the adapted cellular metabolism of the production microorganism. Glucose acts as an exclusive energy and carbon source, and lactose as a substrate for the biosynthesis.
14. The LNFP-l/2’-FL is released from the E. coli K-12 into the fermentation broth. At the end of the fermentation, the E. coli K-12 is removed by filtration (see Diagram 2).
15. Stage 2 of the production process involves a series of purification and isolation steps to generate the final high-purity ingredient (see Diagram 2).
16. The certificates of analysis for the raw materials and the processing aids used in the manufacture of the novel food are provided. D-glucose, D-lactose, D-sucrose and glycerol are food grade. The ‘glucose’ contains ≥ 99.5% glucose. The ‘lactose’ contains ≥ 99% lactose with lactulose (0.05% maximum) and protein (0.2% maximum). The ‘sucrose’ contains ≥ 71% sucrose with inverted sugar (0.5% maximum). The ‘glycerol’ contains ≥ 98% glycerol with moisture content ≤ 5%.
17. The novel food is produced in compliance with current Good Manufacturing Practice (cGMP) and the principles of Hazard Analysis Critical Control Point (HACCP). Manufacturing by-products, impurities and contaminants from the fermentation broth are monitored. Analysis from different manufacturing batches did not detect significant levels of biogenic amines or amino acids and their metabolites in the final product.
18. The absence of bacteria from the Enterobacteriaceae family (ISO 21528-1:2004, MSZ ISO 21528-2:2004) and residual bacterial DNA (futC assay; IgtA assay; galTK assay; 23S assay: LOQ = 4 µg/kg for all assays) confirms the genetically modified E. coli K-12 is not present in the novel food.
19. Analytical data from six batches of LNFP-l/2'-FL confirmed the presence of very low levels of microbial endotoxins and residual proteins (Table 1) which were not considered to be a safety concern.
Table 1: Batch results for microbial endotoxins and residual proteins in the novel food.
|
Batch Number |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5
|
Batch 6 |
|
Residual endotoxins (EU/mg) # |
0.1398 |
0.0023 |
0.0357 |
0.0107 |
< 0.0025 |
0.0012 |
|
Residual protein by Bradford assay (w/w %) |
< 0.0017 |
< 0.0017 |
0.0091 |
< 0.0017 |
< 0.0017 |
< 0.0017 |
|
Aflatoxin M1 (µg/kg) |
< 0.02 |
< 0.02 |
< 0.02 |
< 0.02 |
NT |
< 0.02 |
# Batch 1 has sterile filtration (0.2 μm) conducted at step 8A only; batches 2 - 5 have sterile filtrations at process steps 5A, 6A, 8A and 9A.
20. The production process has characterised the potential hazards and detailed the corresponding control measures sufficiently.
2.3 Compositional Information
21. Results from six independent batches of the novel food demonstrate that the carbohydrate content consistently meets the proposed specification levels (Table 2).
Table 2: The carbohydrate content of the novel food.
|
Parameter (w/w %) |
Specification |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
Batch 6 |
|
Assay (water-free) – specified saccharides a |
≥ 90.0 |
90.18 |
94.63 |
93.49 |
91.85 |
92.93 |
93.94 |
|
Assay (water-free) – LNFP-I + 2'-FL |
≥ 75.0 |
87.41 |
90.66 |
88.68 |
79.48 |
89.06 |
90.83 |
|
Assay (water-free) – LNFP-I |
≥ 50.0 |
56.12 |
62.91 |
69.50 |
59.01 |
56.68 |
61.03 |
|
Assay (water-free) – 2'-FL |
≥ 15.0 |
31.29 |
27.75 |
19.18 |
20.47 |
32.38 |
29.80 |
|
Lacto-N-tetraose |
≤ 5.0 |
0.63 |
1.55 |
3.27 |
3.21 |
1.81 |
1.01 |
|
3-Fucosyllactose |
≤ 1.0 |
0.03 |
< 0.03 |
< 0.03 |
< 0.03 |
< 0.03 |
< 0.03 |
|
Sum of L-Fucose and 2’-fucosyl-lactitol |
≤ 1.0 |
0.20 |
0.06 |
0.05 |
0.11 |
0.06 |
0.06 |
|
D-Lactose |
≤ 10.0 |
0.42 |
1.33 |
0.66 |
7.84 |
0.77 |
0.80 |
|
Difucosyl-D-lactose |
≤ 2.0 |
0.56 |
0.37 |
0.24 |
0.10 |
0.65 |
0.55 |
|
LNFP-I fructose isomer |
≤ 1.5 |
0.60 |
0.43 |
0.40 |
0.49 |
0.23 |
0.49 |
|
2'-Fucosyl-D-lactulose |
≤ 1.0 |
0.43 |
0.13 |
0.08 |
0.14 |
0.15 |
0.13 |
|
Sum of other carbohydrates |
≤ 6.0 |
2.72 |
1.70 |
1.89 |
2.72 |
3.17 |
1.50 |
|
Water |
≤ 8.0 |
1.09 |
2.31 |
2.30 |
3.83 |
5.34 |
2.15 |
|
Sulphated ash |
≤ 0.5 |
0.08 |
< 0.01 |
< 0.01 |
< 0.01 |
0.10 |
< 0.01 |
|
pH in 5% solution (20°C) |
4.0 – 7.0 |
4.6 |
5.9 |
5.7 |
5.4 |
4.4 |
6.5 |
a Sum of specified saccharides includes LNFP-l, 2'-fucosyllactose, lacto-N-tetraose, difucosyl-D-lactose, 3-fucosyllactose, D-lactose, L-fucose and 2’-fucosyl-lactitol, LNFP-I fructose isomer and 2'-fucosyl-D-lactulose.
22. L-fucose and D-lactose are recognised constituents of human breast milk. Difucosyl-D-lactose, lacto-N-tetraose and 3-fucosyllactose are human milk oligosaccharides. Difucosyl-D-lactose and lacto-N-tetraose are present in the Union List of Novel Foods (Regulation (EU) 2017/2470, as retained in UK law), as food ingredients.
23. The fermentation medium contains minerals and trace elements, with trace metals functioning as co-factors for different enzymes. The results from six independent batches of the novel food demonstrated the filtration and purification steps remove these minerals and trace elements to very low levels (Table 3). Analytical data also confirmed the levels of heavy metals were either below the limit of detection or present in very low levels. These results were not considered to be a safety concern.
Table 3: The content of heavy metals, minerals and trace elements in the novel food.
|
Parameter
|
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
Batch 6 |
|
Arsenic (total) (mg/kg) |
< 0.1 |
< 0.1 |
< 0.1 |
< 0.1 |
0.170 |
< 0.100 |
|
Cadmium (mg/kg) |
< 0.01 |
< 0.01 |
< 0.01 |
< 0.01 |
< 0.001 |
< 0.010 |
|
Lead (mg/kg) |
< 0.01 |
< 0.01 |
0.01 |
< 0.01 |
0.01 |
< 0.01 |
|
Mercury (mg/kg) |
< 0.01 |
< 0.01 |
< 0.01 |
< 0.01 |
< 0.005 |
< 0.010 |
|
Phosphate (% w/w) |
< 0.0010 |
< 0.0005 |
0.0004 |
< 0.0001 |
< 0.006 |
< 0.000584 |
|
Sulphate (% w/w) |
0.11 |
0.0049 |
0.003 |
< 0.0044 |
0.0197 |
0.00619 |
|
Chloride (% w/w) |
0.10 |
0.0435 |
0.0057 |
0.0044 |
0.0206 |
0.012757 |
|
Potassium (% w/w) |
0.0020 |
< 0.001 |
0.002 |
0.001 |
0.015 |
< 0.001 |
|
Sodium (% w/w) |
0.1010 |
0.034 |
0.007 |
0.003 |
0.023 |
0.023 |
|
Calcium (% w/w) |
0.0070 |
< 0.001 |
< 0.001 |
< 0.001 |
0.004 |
< 0.000 |
|
Iron (mg/kg) |
1 |
< 0.5 |
< 1 |
< 0.1 |
< 10 |
< 1 |
|
Copper (mg/kg) |
< 0.1 |
0.2 |
0.3 |
0.1 |
< 0.1 |
< 0.2 |
|
Zinc (mg/kg) |
< 0.1 |
0.2 |
0.3 |
0.1 |
< 0.5 |
< 0.2 |
Ammonium (<0.005 w/w%), manganese (< 0.1 mg/kg), selenium (< 0.05 mg/kg), molybdenum (< 0.2 mg/kg) and nickel (< 0.1 mg/kg) reported values that were below the limit of detection for all batches of the novel food
24. Analytical data concerning the microbiological content from six independent batches of the novel food were reported (Table 4). The results confirm that the novel food consistently meets the proposed specification levels.
Table 4: The microbiological analysis of the novel food.
|
Test Parameter (Specification) |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
Batch 6 |
|
Aerobic mesophilic total plate count (≤ 1,000 CFU/g) |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
|
Enterobacteriaceae (absent in 10g) |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
|
Yeasts (≤ 100 CFU/g) |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
|
Moulds (≤ 100 CFU/g) |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
< 10 |
|
Salmonella (absent in 25 g) |
Absent |
Absent |
Absent |
Absent |
Absent |
Absent |
|
Bacillus cereus (≤ 50 cfu/g) |
< 10 |
< 10 |
< 10 |
< 10 |
NT |
< 10 |
|
Listeria monocytogenes (absent in 25g) |
Absent |
Absent |
Absent |
Absent |
NT |
Absent |
|
Cronobacter spp. (absent in 10g) |
Absent |
Absent |
Absent |
Absent |
NT |
Absent |
NT = not tested
25. Certification was provided to demonstrate that the contract laboratories were accredited to perform these analytical studies. Where in-house analysis was used, full methodology and supporting validation documentation was provided.
26. The data presented indicate the novel food can be consistently produced within the proposed specification.
2.4 Stability
27. The stability of the novel food was assessed under real-time conditions (25oC and 60% relative humidity) in two batches over a period of three years, and one batch over a period of nine months. The three year study provided data covering organoleptic, carbohydrates and water content, and microbiological content, whereas the nine month study reported carbohydrates and water content only. A further one batch of novel food was followed over a period of six months under accelerated conditions (40oC and 70% relative humidity) and reported organoleptic, carbohydrates and water, and microbiological content. All results conformed to the novel food specification.
28. The results from a stability study of powdered infant formula containing the novel food under various environmental conditions (5oC; 25oC and 60% RH; 30oC and 65% RH; 40oC and 70% RH) over a period of 12 months. The results confirmed that the novel food in terms of LNFP-l content and microbiological quality is stable over this time period.
29. Based on its structure, the stability of LNFP-l is anticipated to be similar to those of 2'-FL, LNT, and LNnT, which have been approved as food ingredients in the Union List of Novel Foods, Regulation (EU) 2017/2470, as retained in UK law.
30. The data provided supports the stability of the novel food for a time period of at least 12 months.
2.5 Specification
31. The specification parameters reported in Table 5 were assessed using internationally recognised methods or otherwise determined using internally developed and validated methods.
Table 5: Specification of the novel food.
|
Description |
|
LNFP-I /2′-FL is a purified carbohydrate powder or agglomerate obtained from microbial fermentation with a genetically modified strain of Escherichia coli K-12 DH1 containing at least 75% of lacto-N-fucopentaose I and 2’-fucosyllactose of dry matter |
|
Parameter |
Specification |
Method |
|
Appearance |
Powder, agglomerates, powder with agglomerates |
ISO 6658 |
|
Colour |
White, white to off-white, off-white |
ISO 6658 |
|
Assay (water-free) Specified saccharides a |
≥ 90.0 w/w % |
Glycom method HPLC-13-001, HPLC-13-002, HPAEC-HMO-017 |
|
Assay (water-free) – LNFP-I and 2'-FL |
≥75.0 w/w % |
Glycom method HPLC-13-002 |
|
Assay (water-free) – LNFP-I |
≥ 50.0 w/w % |
Glycom method HPLC-13-002 |
|
Assay (water-free) – 2'-FL |
≥ 15.0 w/w % |
Glycom method HPLC-13-002 |
|
Lacto-N-tetraose |
≤ 5.0 w/w % |
Glycom method HPAEC-HMO-017 |
|
3-Fucosyllactose |
≤ 1.0 w/w % |
Glycom method HPAEC-HMO-017 |
|
Sum of L-Fucose and 2’-fucosyl-lactitol |
≤ 1.0 w/w % |
Glycom method HPAEC-HMO-017 |
|
D-Lactose |
≤ 10.0 w/w % |
Glycom method HPAEC-HMO-017 |
|
Difucosyl-D-lactose |
≤ 2.0 w/w % |
Glycom method HPAEC-HMO-017 |
|
LNFP-I fructose isomer |
≤ 1.5 w/w % |
Glycom method HPLC-13-001 |
|
2'-Fucosyl-D-lactulose |
≤ 1.0 w/w % |
Glycom method HPLC-13-001 |
|
Sum of other carbohydrates |
≤ 6.0 w/w % |
Glycom method HPAEC-HMO-017 |
|
pH in 5% solution (20°C) |
4.0–7.0 |
Ph. Eur. 2.2.3 |
|
Water |
≤ 8.0 w/w % |
Glycom method KF-001 |
|
Ash, sulphated |
≤ 0.5 w/w % |
Ph. Eur. 2.4.14 |
|
Residual protein by Bradford assay |
≤ 0.01 w/w % |
Glycom method UV-001 |
|
Residual endotoxins |
≤ 10 EU/mg |
Ph. Eur 2.6.14 (LAL kinetic chromogenic assay) |
|
Aflatoxin M1 |
≤ 0.025 µg/kg |
LC-MS/MS Internal method Neotron 2015 Rev. |
|
Arsenic |
0.2 mg/kg |
EN 13085; EPA-6020A |
|
Aerobic mesophilic total plate count |
≤ 1,000 CFU/g |
ISO 4833-1 or ISO-4833-2 |
|
Enterobacteriaceae |
Absent in 10g |
ISO 21528-2 or NMKL 144 |
|
Salmonella |
Absent in 25 g |
ISO 6579 or AFNOR BRD 07/11-12/05 |
|
Yeasts |
≤ 100 CFU/g |
ISO 21527-2 |
|
Moulds |
≤ 100 CFU/g |
ISO 21527-2 |
|
Bacillus cereus |
≤ 50 cfu/g |
ISO 7392 |
|
Listeria monocytogenes |
Absent in 25g |
ISO 11290-1 |
|
Cronobacter spp. |
Absent in 10g |
ISO 22964 |
32. The information provided is sufficient for the specification of the LNFP-l/2'-FL, and appropriately characterises the novel food seeking authorisation.
2.6 History of Use
33. There is no evidence for a history of use for LNFP-l as a food ingredient.
34. 2’-FL, which has been manufactured either by chemical synthesis or microbial fermentation, is approved as a food ingredient in the List of Novel Foods (Regulation (EU) 2017/2470, as retained in UK law) and can be added to several food categories in doses up to 1.2 g/L. In addition, 2’-FL, is approved as a food ingredient in Brazil, Malaysia, Singapore, Switzerland, Thailand, and the USA.
35. Human breast milk contains a family of structurally related oligosaccharides, known as human milk oligosaccharides (HMOs), as the third largest solid components (Kunz and Rudloff, 1993; Bode, 2012; Newburg, 2013). The concentrations of HMOs in human colostrum are 20 to 25 g/L, whereas in mature human milk, the concentrations are 5 to 20 g/L (Bode, 2012). A wide variability has been reported in these values, depending on the individual, lactation period and genotype of the mother. Although there are over 140 known HMOs (Urashima et al., 2011; Chen, 2015; Remoroza et al., 2020), the five most abundant HMOs, on average, account for nearly half of the oligosaccharide fraction by mass. These are 2'-FL and LNFP-l, which are the novel food, and lacto-N-difucohexaose l (LNDFH-l), LNT, and 3-fucosyllactose (3-FL) (Thurl et al., 2017; Molnar-Gabor et al., 2019).
36. A literature review of the quantitative data for LNFP-l in breast milk from twenty four publications revealed that average levels vary from 0.18 g LNFP-I/L (Smilowitz et al., 2013) to 4.47 g LNFP-l/L (Elwakiel et al., 2018). Using the reported standard deviations, the values reported by Elwakiel et al. (2018) also represented the highest extrapolated 95% confidence limit (CL) at 5.75 g LNFP-l/L. The estimated highest mean and highest 95% CL intake levels of LNFP-I from human breast milk are reported in Table 6.
Table 6: Highest Intakes of LNFP-l from 800ml and 1200ml of Breast milk for 6.7kg Infant a
|
Human Milk Oligosaccharide |
Estimated highest intake for 800ml b milk (mg/kg bw/day) |
Estimated highest intake for 1200ml b milk (mg/kg bw/day) |
|
LNFP-l |
|
|
|
Highest Mean (based on 4.47 g/L) |
534 |
801 |
|
Highest 95% CL (based on 5.57 g/L |
665 |
998 |
a EFSA SC, 2012
b EFSA NDA Panel, 2013
37. This systematic review also revealed that average levels of 2’-FL in human breast milk varied from 0.68 g 2’-FL/L (van Niekerk et al, 2014) to 7.23 g 2’-FL/L (Gabrielli et al, 2011). Using the reported standard deviations, the values reported by Gabrielli et al. (2011) also represented the highest extrapolated 95% CL at 14.01 g 2’-FL/L. The estimated highest mean and highest 95% CL intake levels of 2’-FL from human breast milk are reported in Table 7.
Table 7: Highest Intakes of 2’-FL from 800ml and 1200ml of Breast milk for 6.7kg Infant a
|
Human Milk Oligosaccharide |
Estimated highest intake for 800ml b milk (mg/kg bw/day) |
Estimated highest intake for 1200ml b milk (mg/kg bw/day) |
|
2’-FL |
|
|
|
Highest Mean (based on 7.23 g/L) |
863 |
1,295 |
|
Highest 95% CL (based on 14.01 g/L) |
1,673 |
2,509 |
a EFSA SC, 2012
b EFSA NDA Panel, 2013
38. The history of use does not indicate any further areas for evaluation.
2.7 Proposed Use and Anticipated Intake
39. Infants, children, and adults, including pregnant and lactating women, are identified as the target population of the novel food. The proposed food categories and maximum use levels are listed in Table 8.
Table 8: Food Categories and Use Levels for LNFP-I from the novel food.
|
Food Category Name |
Proposed Maximum Use Level (expressed as LNFP-I) |
|
Dairy Products and Analogues |
|
|
Unflavoured pasteurised and unflavoured sterilised (including UHT) milk |
1.0 g/L |
|
Unflavoured fermented milk-based products |
1.0 g/L (beverages) 2.0 g/kg (products other than beverages) |
|
Flavoured fermented milk-based products including heat-treated products |
1.0 g/L (beverages) 10.0 g/kg (products other than beverages) |
|
Bakery wares |
|
|
Fine bakery wares. Cereal bars only |
10.0 g/kg |
|
Foods for Special Groups (FSG) |
|
|
Foods for infants and young children |
|
|
Infant formula as defined in Regulation (EU) No 609/2013 |
1.5 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
|
Follow-on formula as defined in Regulation (EU) No 609/2013 |
1.5 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
|
Processed cereal-based food and baby food for infants and young children as defined in Regulation (EU) No 609/2013 |
1.0 g/L (beverages) in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer 8.33 g/kg (products other than beverages) |
|
Milk-based drinks and similar products intended for young children |
1.2 g/L (beverages) in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer 10 g/kg (products other than beverages) |
|
Foods for special medical purposes (FSMP) defined in Regulation (EU) No 609/2013 |
|
|
Foods for special medical purposes defined in Regulation (EU) No 609/2013 |
In accordance with the particular nutritional requirements of the persons for whom the products are intended |
|
Total diet replacement for weight control as defined in Regulation (EU) No 609/2013 |
|
|
Total diet replacement for weight control as defined in Regulation (EU) No 609/2013 |
2.0 g/L (beverages) 20.0 g/kg (products other than beverages) |
|
Beverages |
|
|
Flavoured drinks (excluding cola-type drinks) |
1.0 g/L |
|
Food supplements |
|
|
Food supplements (infants and young children) |
1.5 g/day |
|
Food supplements (other children, adolescents and adults) |
3.0 g/day |
40. The anticipated intake for LNFP-l in children up to the age of 16 weeks is estimated to be 390 mg/kg body weight/day, equivalent to 2.09 g/day for a 6.7 kg infant. This value was calculated from the use of LNFP-l in infant formula (1.5 g/L) at a high consumption level of 260 ml/kg body weight/day, as established by the EFSA Scientific Committee (EFSA SC, 2017). This value does not exceed the estimated intake for LNFP-l from breast milk (see Table 6).
Table 9: Estimated daily intake of LNFP-l and 2’-FL from proposed food uses.
|
Population |
Mean Intake of LNFP-I (mg/kg bw/day) |
Mean Intake of 2’-FL (mg/kg bw/day) |
High Level a Intake of LNFP-I (mg/kg bw/day) |
High Level a Intake of 2’-FL (mg/kg bw/day) |
|
Infants (≤ 11 months) |
52 – 248 |
22 – 106 |
155 – 462 |
66 – 198 |
|
Young children (12 to 35 months) |
26 – 123 |
11 – 53 |
49 – 423 |
21 – 181 |
|
Other children (3 to 9 years) |
7 – 34 |
3 – 15 |
18 – 59 |
8 – 25 |
|
Adolescents (10 – 17 years) |
2 – 14 |
0.9 – 6 |
8 – 29 |
3.4 – 12 |
|
Adults (18 – 64 years) |
1 – 8 |
0.4 – 3.4 |
8 – 21 |
3.4 – 9 |
|
Pregnant and Lactating Women |
3 – 7 |
1.3 – 3 |
8 – 14 |
3.4 – 6 |
|
Elderly (65 – 74 years) |
1 – 5 |
0.4 – 2.1 |
5 – 14 |
2.1 – 6 |
|
Very Elderly (≥ 75 years) |
1 – 6 |
0.4 – 2.6 |
6 – 10 |
0.4 – 4 |
a Results are not presented that were not statistically reliable (n < 60).
41. An intake assessment using the summary statistics of consumption from the dietary surveys in the EFSA Comprehensive database was conducted by matching the proposed conditions of use with the FoodEx2 categories. The corresponding use level of 2’-FL is calculated as 0.428 x LNFP-l level (this factor is derived from the average content of LNFP-I and 2’-FL determined by analysis in Table 2: 2’-FL÷ LNFP-l = 26.5 / 61.9 = 0.428). Estimated mean and high-level intakes of LNFP-l and 2’-FL from the proposed conditions of use for each sub-population are presented in Table 9.
42. The analysis indicates that the higher intakes in the infant and young children groups are expected given the relatively high intake of foods and beverages on a body weight basis compared to the other population groups, where the intake levels are significantly lower. The wide range of high intake levels for LNFP-l in infants and young children represent the variability in the national survey data, and the assumption that all the proposed foods will be consumed at the maximum proposed intake levels, but this was considered to be extremely unlikely.
Table 10: Highest Intakes of LNFP-l and 2'-FL from 800ml and 1200ml of Breast Milk versus Highest Level of Exposure from Proposed Uses for 6.7kg Infant
|
Human Milk Oligosaccharide |
Estimated Daily Intake for 800ml a (mg/kg bw/day) |
Estimated Daily Intake for 1200ml a (mg/kg bw/day) |
|
LNFP-l |
|
|
|
Highest Mean (based on 4.47 g/L b) |
534 |
801 |
|
Highest 95% CL (based on 5.57 g/L b) |
665 |
998 |
|
Highest Mean Consumption from Proposed Uses b |
248 |
462 |
|
|
|
|
|
2-‘FL |
|
|
|
Highest Mean (based on 7.23 g/L b) |
863 |
1,295 |
|
Highest 95% CL (based on 14.01 g/L b) |
1,673 |
2,509 |
|
Highest 95th Percentile Consumption from Proposed Food Uses c |
106 d |
198 d |
a EFSA NDA Panel, 2013
b Calculated using quantitative analytical data for oligosaccharides in breast milk
c Estimated intake for all infants.
d 2’-FL consumption is calculated from LNFP-l intakes based on the average values in table 2.c.2-1, i.e., 26.5/61.9 = 0.428.
43. A comparison of the estimated mean and high level intake of LNFP-l/2'-FL (based on all infants) against the highest mean and upper confidence limit exposures from breast milk for LNFP-l and 2’-FL on a body weight basis (EFSA NDA Panel, 2013), are shown in Table 10. This data indicates that the estimated intakes of LNFP-l/2’-FL, in the most sensitive population group (infants), are not expected to exceed the levels found in human breast milk, for which there is a history of safe use.
44. The use level for LNFP-l/2'-FL in food supplements is 1.5 g/day for infants and young children, and 3 g/day for all other population sub-groups. It was noted that food supplements are not intended to be used if other foods with the novel food are consumed on the same day. For infants and young children, food supplements are not intended to be used if breast milk or other foods with added LNFP-l/2'-FL are consumed on the same day.
Table 11: Estimated daily intake of LNFP-l from food supplements
|
Proposed use level (g/day) |
Population Group |
Mean Body Weight (kg) a |
Estimated intake (mg/kg bw/day) b |
|
1.5 |
Infants (≤ 11 months) |
6.7 |
224 |
|
1.5 |
Young children (12 to 35 months) |
11.9 |
126 |
|
3.0 |
Other children (3 to 9 years) Adolescents (10 – 17 years) Adults (18 – 64 years) Elderly (65 – 74 years) Very Elderly (≥ 75 years) |
23.1 43.4 73.9 76.0 71.2 |
130 69 41 39 42 |
a EFSA SC 2012
b Calculation: [Proposed Use Level (g/day) / Mean Body Weights] x 1,000.
45. The estimated intake for LNFP-l/2'-FL in food supplements on a body weight basis for all population groups at the proposed use levels is presented in Table 11.
46. The data in Table 11 indicates that the sub-population with the highest consumption level is expected to be infants. Based on the proposed infant food supplement use at 1.5 g/day, the high level intake of LNFP-l and 2'-FL is estimated as 224 mg/kg body weight/day and 96 mg/kg body weight/day, respectively. These values are not be expected to exceed the levels found in human breast milk (Table 10), for which there is a history of safe use.
2.8 Absorption, Distribution, Metabolism and Excretion (ADME)
47. The LNFP-l and 2'-FL oligosaccharides in the novel food have the same structure as their naturally occurring counterparts in human breast milk.
48. Most human milk oligosaccharides are reported to undergo limited oral absorption intact. Human milk oligosaccharides do not undergo significant digestion in the upper gastrointestinal tract but can undergo fermentation in the colon. Human milk oligosaccharides are predominantly excreted unchanged in the faeces, with a small proportion excreted unchanged in the urine.
49. The absorption of LNFP-l and 2'-FL from consumption of the novel food is not expected to differ from the intake of human milk oligosaccharides following infant consumption of breast milk. Therefore, this was not expected to pose a safety concern for infants or other age groups.
50. Committee members noted that oligosaccharides are usually considered prebiotic and can cause bloating in high doses. Consequently, it was highlighted that risk managers may wish to consider whether there was a need for foods containing LNFP-l/2’-FL to be labelled on this basis.
51. The ADME of human milk oligosaccharides are well understood and the information does not indicate any further areas of concern.
2.9 Nutritional information
52. The novel food is mainly composed of the oligosaccharides, LNFP-l and 2’-FL, which are structurally identical to their naturally occurring counterparts in human breast milk Consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
2.10 Toxicological information
53. Toxicological studies were performed with LNFP-l/2'-FL to support the safety assessment of the novel food. The respective study reports are unpublished and claimed as proprietary data. They were considered essential in the assessment of the safety of the novel food and were reviewed by the ACNFP.
54. In vitro genotoxicity testing of LNFP-l/2’-FL was conducted under Good Laboratory Practice (GLP) conditions and according to the OECD guidelines: in vitro bacterial reverse mutation test (OECD TG 471) and in vitro mammalian cell micronucleus test (OECD TG 487). This approach is recommended by the UK Committee on Mutagenicity, and is also the basis of guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283, as retained in UK law.
55. The in vitro bacterial reverse mutation test (Gilby, 2020a) demonstrated that LNFP-l/2’-FL is non-mutagenic, in the absence or presence of metabolic activation.
56. The in vitro mammalian cell micronucleus test (Gilby, 2020b) demonstrated that LNFP-l/2’-FL is non-clastogenic and non-aneugenic in the absence or presence of metabolic activation.
57. The results from these in vitro studies support the conclusion that the novel food is not genotoxic.
58. A Repeated Dose 90-Day Oral Toxicity Study in Rodents (Stannard, 2020) was conducted under GLP conditions according to OECD TG 408 guidelines as recommended by the Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283, as retained in UK law. The aim of the study was to identify any adverse effects following the consumption of LNFP-l/2'-FL.
59. In this 90-day feeding study, each group consisted of 10 female and 10 male rats which were fed 0 (control – vehicle only [water]), 1,000, 3,000 or 5,000 mg/kg bw/day of LNFP-l/2'-FL by oral gavage. A reference control group consisting of the same number of animals was fed oligofructose (5,000 mg/kg bw/day). Additionally, satellite groups consisting of 5 female rats and 5 male rats for the control and high dose treatment, and the reference control treatment were used as recovery groups.
60. No deaths, test item-related clinical abnormalities, ocular changes, or differences in food consumption and bodyweight between test groups were reported. There were no statistically significant dose dependent changes in haematology, blood chemistry, urinalysis, serum hormone levels, or organ weights. In addition, no dose related abnormalities were noted during the necropsy or histopathological evaluation. Therefore, the no observable adverse effect level (NOAEL) for LNFP-l/2'-FL was considered to be the high-dose of 5,000 mg/kg bw/day.
2.11 Allergenicity
61. The protein content of the novel food is reported as < 0.0017% w/w. The potential allergenicity of the introduced proteins expressed in E. coli K-12 (Allergen Online tool, version 20 – University of Nebraska) was assessed. None of the proteins was predicted to be an allergen.
62. The novel food is unlikely to trigger allergic reactions in the target population under the proposed conditions of use.
3. Discussion
63. The novel food is a mixture of human identical milk oligosaccharides containing ≥ 75.0% dry weight of LNFP-l/2'-FL, which are manufactured by microbial fermentation using a genetically modified strain of Escherichia coli K-12. The total saccharide content of the novel food is up to 94% dry weight including the presence of other saccharides present in smaller quantities.
64. LNFP-l/2'-FL is intended to be used in dairy products and analogues, bakery wares, beverages, foods for infants and young children, foods for special medical purposes, total diet replacement for weight control, and food supplements. Infants, children, and adults, including pregnant and lactating women, are identified as the target population of the novel food.
65. Analyses confirm that the novel food is structurally identical to the LNFP-l and 2'-FL found in human breast milk. The oligosaccharide 2'-FL is authorised as a novel food in the UK, EU and other parts of the world. Prior exposure to LNFP-l relates solely to breastfeeding infants as there is no recognised history of use for this milk oligosaccharide as an ingredient in foods or food supplements.
66. In the Repeated Dose 90-Day Oral Toxicity Study in Rodents, the NOAEL for LNFP-l/2'-FL was 5,000 mg/kg bw/day, the highest dose tested. When this NOAEL is compared with the highest estimated exposure in each population category, the margins of exposure range from 7 to 357. Given that the LNFP-l and 2'-FL in the novel food are equivalent to LNFP-l and 2'-FL found in human breast milk, these margins of exposure are acceptable with respect to the highest estimated daily intakes in the intended population.
67. Moreover, the anticipated daily intake of the novel food in all population groups, including children up to the age of 16 weeks using infant formula alone, is not expected to exceed the highest intake level of LNFP-l/2'-FL in breastfed infants on a body weight basis.
68. The use level of LNFP-l/2'-FL in food supplements (1.5 g/day for infants and young children, and 3 g/day for all other population sub-groups) is not expected to exceed the highest intake level of LNFP-l/2'-FL in breastfed infants on a body weight basis. Food supplements are not intended to be used if other foods containing the novel food, including breast milk or other foods for infants and young children, are consumed on the same day. It is noted that the concurrent use of LNFP-l/2'-FL in foods and food supplements by adolescents and adults would not be expected to exceed the intake levels of LNFP-l/2'-FL in breastfed infants on a bodyweight basis.
4. Conclusions
69. The ACNFP have undertaken the assessment of LNFP-l/2'-FL and concluded that the composition of the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health.
70. These conclusions were based on the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
- annexes to the dossier which relate to the identity of the novel food, the production process, composition, stability, history of use, and the anticipated intake of the novel food.
- bacterial reverse mutation test (Gilby, 2020a [unpublished]), in vitro micronucleus test (Gilby, 2020b [unpublished]) and 90-day repeat dose feeding study with the novel food (Stannard, 2020 [unpublished]) including the summary table of statistically significant observations in the toxicity studies (Appendix B.3).
71. With thanks to the members of the ACNFP during the course of the assessment who were; Dr Camilla Alexander White, Dr Anton Alldrick, Alison Austin, Dr Mark Berry, Professor Dimitris Charalampopoulos, Professor Susan Duthie, Professor Susan Fairweather-Tait, Professor Paul Frazer, Dr Hamid Ghoddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw Jones, Dr Ray Kemp, Dr Elizabeth Lund, Nichola Lund, Dr Rohini Manuel, Emeritus Professor Harry McArdle, Rebecca McKenzie, Professor Clare Mills, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield and Professor Bruce Whitelaw.
5. References
Bode L, 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22(9), 1147-1162. https://doi.org/10.1093/glycob/cws074
Chen X, 2015. Human milk oligosaccharides (HMOS): structure, function, and enzyme-catalyzed synthesis. Advances in Carbohydrate Chemistry and Biochemistry, 72, 113-190. https://doi.org/10.1016/bs.accb.2015.08.002
EC, 2017. Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods.
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2469
EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2011. Scientific Opinion on Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use (Question no EFSA-Q-2009-00521, adopted: 25 May 2009). EFSA Journal, 9(6):2193 [54 pp.].
https://doi.org/10.2903/j.efsa.2011.2193.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal, 11(10):3408 [103 pp.].
https://doi.org/10.2903/j.efsa.2013.3408.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2021. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283 (Revision 1). Published 26 March 2021. https://doi.org/10.2903/j.efsa.2011.2170
EFSA Scientific Committee, 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal, 10(3):2579. [32 pp.]
https://doi.org/10.2903/j.efsa.2012.2579.
EFSA Scientific Committee, 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (Question no EFSA-Q-2016-00489, adopted: 26 April 2017). EFSA Journal, 15(5), 4849 [58 pp.].
https://doi.org/10.2903/j.efsa.2017.4849.
EU (2015) Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32015R2283
Elwakiel M, Hageman JA, Wang W, Szeto IM, van Goudoever JB, Hettinga KA and Schols HA, 2018. Human milk oligosaccharides in colostrum and mature milk of Chinese mothers: Lewis positive secretor subgroups. Journal of Agricultural and Food Chemistry, 66(27), 7036-7043. https://doi.org/10.1021/acs.jafc.8b02021
Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C and Coppa GV, 2011. Preterm milk oligosaccharides during the first month of lactation. Pediatrics, 128(6), e1520-e1531. https://doi.org/10.1542/peds.2011-1206
Gilby B, 2020a [unpublished]. Dated: 23 January 2020. Covance CRS Limited, Huntingdon, Cambridgeshire, UK. Study Title: Report. Lacto-N-fucopentaose I / 2’-Fucosyllactose mixture (LNFP-I / 2’-FL): Bacterial reverse mutation test. (Covance Study No: YP48JX).
Gilby B, 2020b [unpublished]. Dated: 19 February 2020. Covance CRS Limited, Huntingdon, Cambridgeshire, UK. Study Title: Report. Lacto-N-fucopentaose I / 2’-Fucosyllactose mixture (LNFP-I / 2’-FL): In Vitro micronucleus test in human lymphocytes. (Covance Study No: QR38KG).
Kunz C and Rudloff S, 1993. Biological functions of oligosaccharides in human milk. Acta Paediatrica, 82(12), 903-912.
https://doi.org/10.1111/j.1651-2227.1993.tb12597.x
Molnar-Gabor D, Hederos MJ, Bartsch S and Vogel A, 2019. Emerging field – Synthesis of complex carbohydrates. Case study on HMOs (Chapter 2.5). In: Industrial enzyme applications. Eds Vogel A and, May O. Wiley-VCH Verlag GmbH, Weinheim, Germany, 179-201. https://doi.org/10.1002/9783527813780.ch2_5
Newburg DS, 2013. Glycobiology of human milk. Biochemistry (Moscow), 78, 771-785 (Russian edition "Biokhimiya, 778, 990-1007").
https://doi.org/10.1134/s0006297913070092
OECD (Organisation for Economic Co-operation and Development), 1997. Bacterial reverse mutation test. In OECD guidelines for the testing of chemicals. OECD guideline No 471 (updated & adopted: 21 July 1997). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264071247-en
OECD (Organisation for Economic Co-operation and Development), 1998. OECD principles of good laboratory practice. Series on principles of good laboratory practice and compliance monitoring, No. 1 (ENV/MC/CHEM(98) 17). Paris, France: Organisation for Economic Co-operation and Development (OECD), Environment
Directorate, Chemicals Group and Management Committee.
https://doi.org/10.1787/9789264078536-en
OECD (Organisation for Economic Co-operation and Development), 2016. In vitro mammalian cell micronucleus test. In OECD guidelines for the testing of chemicals. OECD guideline No 487 (updated & adopted: 29 July 2016). Paris, France: Organisation for Economic Cooperation and Development (OECD).
https://doi.org/10.1787/9789264264861-en
OECD (Organisation for Economic Co-operation and Development), 2018. Repeated dose 90-day oral toxicity study in rodents. In OECD guidelines for the testing of chemicals. OECD guideline No 408 (updated and adopted 27 June 2018). Paris, France: Organisation for Economic Cooperation and Development (OECD).
https://doi.org/10.1787/9789264070707-en
Remoroza CA, Liang Y, Mak TD, Mirokhin Y, Sheetlin SL, Yang X, San Andres JV, Power ML and Stein SE, 2020. Increasing the coverage of a mass spectral library of milk oligosaccharides using a hybrid-search-based bootstrapping method and milks from a wide variety of mammals. Analytical Chemistry, 92(15), 10316-10326.
https://doi.org/10.1021/acs.analchem.0c00342
Smilowitz JT, O'Sullivan A, Barile D, German JB, Lönnerdal B and Slupsky CM, 2013. The human milk metabolome reveals diverse oligosaccharide profiles. Journal of Nutrition, 143(11), 1709-1718. https://doi.org/10.3945/jn.113.178772
Stannard DR, 2020 [unpublished]. Dated 25 August 2020. Covance CRS Limited, Suffolk, UK. Study Title: Final report. Lacto-N-fucopentaose I / 2’-Fucosyllactose mixture (LNFP-I / 2’-FL): 90-Day toxicity study in the neonatal CRL:CD(SD) rat by oral (gavage) administration followed by a 4-week recovery period. (Study No: FC89HQ).
Thurl S, Munzert M, Boehm G, Matthews C and Stahl B, 2017. Systematic review of the concentrations of oligosaccharides in human milk. Nutrition Reviews, 75(11), 920-933. https://doi.org/10.1093/nutrit/nux044
Townsend S, Caubilla Barron J, Loc-Carrillo C, Forsythe, S (2007). The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiology, 24(1), 67-74. https://doi.org/10.1016/j.fm.2006.03.009
Urashima T, Fukuda K, Kitaoka M, Ohnishi M, Terabayashi T and Kobata A, 2011. Milk oligosaccharides. Nova Biomedical Books, New York, Nutrition and diet research progress.
van Niekerk E, Autran CA, Nel DG, Kirsten GF, Blaauw R and Bode L, 2014. Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. The Journal of Nutrition, 144(8), 1227-1233
https://doi.org/10.3945/jn.113.187799
Abbreviations
| 2'-FL | 2'-fucosyllactose |
| ACNFP | Advisory Committee on Novel Foods and Processes |
| ADME | Absorption, Distribution, Metabolism and Excretion |
| AFNOR | Association Francaise de Normalisation |
| bw | body weight |
| CL | confidence limit |
| CAS | Chemical Abstracts Service |
| CFU | Colony Forming Unit |
| cGMP | Current Good Manufacturing Practice |
| DNA | Deoxyribonucleic acid |
| EC | European Commission |
| EFSA | European Food Safety Agency |
| EPA | Environmental Protection Agency |
| EU | European Union |
| EU/mg | Endotoxin units per milligram |
| GLP | Good Laboratory Practice |
| HACCP | Hazards Analysis and Critical Control Points |
| HPAEC | High-performance anion exchange chromatography |
| HPLC | High-performance liquid chromatography |
| ISO | International Organisation for Standardization |
| KF | Karl Fisher |
| LC-MS/MS | Liquid chromatography-tandem mass spectroscopy |
| LNFP-l | lacto-N-fucopentaose l |
| LOQ | limit of quantification |
| NMKL | Nordisk Metodikkomite for Levnedsmidler |
| NOAEL | No Observable Adverse Effect Level |
| NT | Not tested |
| OECD | Organisation for Economic Co-operation and Development |
| Ph. Eur. | European Pharmacopoeia |
| RT | retention time |
| UHT | ultra-high temperature |
| UV | ultra-violet |