Committee Advice on the safety of Magnesium L-threonate as a novel food for use in food supplements
On this page
Skip the menu of subheadings on this page.Reference Number RP956
Assessment finalised: 18th of September 2023
Summary
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in April 2021 from AIDP, USA (“the applicant”) for the authorisation of magnesium L-threonate monohydrate as a novel food.
The novel food is magnesium L-threonate monohydrate which is intended to be used as a source of magnesium in food supplements. Magnesium L-threonate monohydrate is manufactured via a chemical synthetic process, isolated, purified and then dried to a powder.
This new application is seeking to use the novel food within the food category: food supplement.
To support the FSA and FSS in their evaluation of the application, the Advisory Committee on Novel Foods and Processes (ACNFP) were asked to review the safety dossier and supplementary information provided by the applicant. Please note the Committee did not consider any potential health benefits or claims arising from consuming the food, as the focus of the novel food assessment is to ensure the food is safe, and not putting consumers at a nutritional disadvantage.
The Committee concluded that the applicant had provided sufficient information to assure the novel food, magnesium L-threonate monohydrate, was safe under the proposed conditions of use. The anticipated intake levels and the proposed use in food supplements was not considered to be nutritionally disadvantageous.
1. Introduction
1. The ACNFP assessed the food safety risks of magnesium L-threonate monohydrate and its production under the proposed uses, in line with Article 7 of assimilated Commission Implementing Regulation (EU) 2017/2469. The regulatory framework and the technical guidance put in place by the European Food Safety Agency (EFSA) for full novel food applications is retained as the basis and structure for the assessment (EFSA NDA Panel, 2021).
2. In April 2021, AIDP, USA (“the applicant”) submitted a full novel food application for the authorisation of magnesium L-threonate monohydrate. The novel food is a water soluble white to off-white powder which is manufactured via a chemical synthetic process. Magnesium L-threonate monohydrate is intended to be used as a source of magnesium in food supplements.
3. Following the review by the ACNFP in September 2022, further information was requested from the applicant concerning the identity of the novel food, the production process, the proposed uses, ADME and nutritional information on magnesium L-threonate monohydrate, in order to address information gaps in the initial dossier. The final advice from the Committee was agreed at the 162nd meeting, allowing the FSA and FSS to complete the risk assessment.
4. The Committee advice document (CAD) outlines the conclusions of the ACNFP on the safety of magnesium L-threonate monohydrate as a novel food.
2. Assessment
2.1 Identity of the novel food
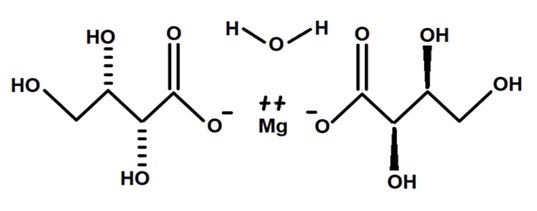
5. The novel food, magnesium L-threonate monohydrate (Diagram 1), is classified as a purified chemical substance and characterised by the following information:
IUPAC name Magnesium (2R,3S)-2,3,4-trihydroxybutanoate monohydrate
CAS number 500304-76-7
Molecular weight 312.51 g/mol
Molecular formula C8H16MgO11
Figure 1: The structural formula of magnesium L-threonate monohydrate.
6. Magnesium L-threonate monohydrate is a water soluble white to off-white powder which is manufactured via a chemical synthetic process. The content of the novel food is 98 – 102% magnesium L-threonate monohydrate.
7. Confirmation to support the identification of the novel food as magnesium L-threonate monohydrate was provided by elemental analysis, inductively coupled plasma optical emission spectroscopy (ICP-OES) and high-performance liquid chromatography (HPLC).
8. Fourier Transform infrared spectroscopy (FT-IR) analysis ensures that the novel food is magnesium L-threonate monohydrate.
9. Information to support this characterisation was provided for five batches of magnesium L-threonate monohydrate.
2.2 Production Process
10. The production process for magnesium L-threonate monohydrate is a two-step chemical synthetic process. In the first step, ascorbic acid and calcium carbonate are reacted in the presence of hydrogen peroxide to the form calcium L-threonate as an intermediate.
11. In the second step, the calcium in the calcium L-threonate is replaced with magnesium by the addition of magnesium carbonate and oxalic acid.
12. The crude product is then isolated, purified, and dried to a powder to yield magnesium L-threonate monohydrate.
13. Information on the acceptance criteria for the raw materials and processing aids was provided. The purity criteria for the reagents used in the manufacture of magnesium L-threonate monohydrate are listed in Table 1.
Table 1: Purity criteria for reagents used to synthesize the novel food
|
Raw material |
Purity |
|
Calcium carbonate |
≥ 98% |
|
Ascorbic acid |
≥ 99% |
|
Hydrogen peroxide, aqueous |
≥ 27.5% |
|
Hydrated basic magnesium carbonate, heavy |
40 – 44% magnesium oxide by assay * |
|
Oxalic acid |
≥ 99.6% |
* Complies with European Pharmacopoeia
14. The manufacturing plant is registered with Food and Drug Administration (FDA) and Californian state authorities. The novel food is produced in compliance with current Good Manufacturing Practice (cGMP) and implements Hazard Analysis and Critical Control Point (HACCP) principles.
15. The presence of oxalic acid residues in the novel food are controlled by monitoring the pH of the reaction solution and ensuring that the content of oxalic acid does not exceed the critical limit of 0.5% for each batch of magnesium L-threonate monohydrate.
16. The production process has characterised the potential hazards and the corresponding control measures are appropriate.
2.3 Compositional information
17. Results from five independent batches of magnesium L-threonate monohydrate demonstrated that the novel food consistently meets the specification levels (Table 2).
Table 2. Compositional Analysis of the novel food
|
Test Parameter |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
|
Appearance |
Conforms |
Conforms |
Conforms |
Conforms |
Conforms |
|
Odour and Taste |
Conforms |
Conforms |
Conforms |
Conforms |
Conforms |
|
Solubility |
Conforms |
Conforms |
Conforms |
Conforms |
Conforms |
|
Colour of solution |
Conforms |
Conforms |
Conforms |
Conforms |
Conforms |
|
Bulk density (g/ml) |
0.68 |
0.72 |
0.77 |
0.67 |
0.68 |
|
Particle size NLT 90% thru a US # 20 NMT 60% thru a US # 200 |
100.0% 25% |
100.0% 31% |
100.0% 30% |
100.0% 30% |
100.0% 32% |
|
Identification |
Conforms |
Conforms |
Conforms |
Conforms |
Conforms |
|
Loss on drying (%) |
0.4 |
0.3 |
0.2 |
0.3 |
0.1 |
|
Assay (%) |
100.6 |
100.5 |
100.7 |
100.6 |
100.5 |
|
Magnesium (%) |
7.7 |
7.7 |
7.7 |
7.7 |
7.7 |
|
L-Threonate (%) |
ND |
ND |
ND |
ND |
ND |
|
Ethanol residues (ppm) |
6 |
138 |
148 |
147 |
131 |
|
pH [USP (1% in H2O)] |
6.3 |
6.4 |
6.4 |
6.4 |
6.4 |
|
Arsenic (ppm) |
0.011 |
0.014 |
0.012 |
0.03 |
0.019 |
|
Mercury (ppm) |
0.002 |
0.007 |
0.006 |
0.001 |
0.0033 |
|
Lead (ppm) |
0.042 |
0.044 |
0.030 |
0.036 |
0.028 |
|
Cadmium (ppm) |
0.005 |
0.004 |
0.008 |
0.003 |
< 0.001 |
|
Total plate count (CFU/g) |
10 |
10 |
10 |
< 10 |
10 |
|
Yeast and mould (CFU/g) |
10 |
< 10 |
< 10 |
< 10 |
< 10 |
|
E. coli (Absent in 1g) |
Absent |
Absent |
Absent |
Absent |
Absent |
|
Salmonella (Absent in 25g) |
Absent |
Absent |
Absent |
Absent |
Absent |
Assay = [(% magnesium + % threonate) x (312.51 / 294.50)] + % water
NLT = not less than; NMT = not more than; ND = not determined; ppm = parts per million; CFU = colony forming units
18. The assay calculation for magnesium L-threonate monohydrate requires three parameters: magnesium, L-threonate and loss on drying content. As the L-threonate content was not provided (Table 2), further analytical data was requested for magnesium, L-threonate monohydrate and loss on drying (Table 3).
Table 3. Magnesium, L-threonate and loss on drying content for magnesium L-threonate monohydrate
|
Test Parameter |
Batch 6 |
Batch 7 |
Batch 8 |
|
Magnesium (%) |
7.7% |
7.8% |
7.8% |
|
L-threonate (%) |
85.4% |
86.2% |
85.3% |
|
Loss on drying (%) |
0.2% |
0.2% |
0.2% |
|
Assay (%) |
99.0% |
100.0% |
99.0% |
Assay = [(% magnesium + % threonate) x (312.51 / 294.50)] + % water
19. An assessment was conducted in accordance with the EFSA Guidance on technical requirements for regulated food and feed product applications (EFSA, 2021) to establish the presence of small particles including nanoparticles in the novel food. A water solubility test, following OECD TG 105 guidelines, confirmed that the novel food exceeded the decision criteria for solubility (> 33.3 g/L), as described in the guidance. Therefore, further evaluation for the presence of nanoparticles in magnesium L-threonate monohydrate is not required.
20. Certification was provided to demonstrate that the contract laboratories were accredited to perform these analytical studies. Where in-house analysis was utilised, full methodology and supporting validation documentation was provided.
21. The data presented indicate the novel food can be consistently produced within the proposed specification.
2.4 Stability
22. Three batches of magnesium L-threonate monohydrate were assessed in a real-time stability study at 25 +/- 2oC for 36 months and a 6-month accelerated stability study. The physicochemical, biochemical, and microbiological parameters complied with the novel food specification limits confirming magnesium L-threonate monohydrate is stable under these conditions.
23. An additional 36-month real-time stability study for two batches of magnesium L-threonate monohydrate, following the same parameters, confirmed the novel food is stable under these conditions.
24. The data provided supports the stability of the novel food for up to 36 months.
2.5 Specification
25. The specification parameters for the novel food (Table 4) were assessed using internationally recognised methods or are otherwise determined using internally developed and validated methods.
Table 4: Specification for magnesium L-threonate monohydrate
|
Parameter |
Specification |
Method |
|
Appearance |
White powder |
Visual |
|
Odour and Taste |
Characteristic |
Organoleptic |
|
Solubility |
Water soluble |
Visual (1% at 25°C) |
|
Colour of solution |
Clear |
Visual (1% solution) |
|
Bulk density |
> 0.4 g/ml |
Loose fill in graduated cylinder |
|
Particle size |
NLT 90% thru a US # 20 MMT 60% thru a US # 200 |
Ro Tap sieve shaker (3 min.) Ro Tap sieve shaker (3 min.) |
|
Identification |
Conforms to standard |
FTIR |
|
Loss on drying |
≤ 5.0% |
105°C, 4 hours |
|
Assay |
98-102% |
Calculation |
|
Magnesium |
7.2 to 8.3% (mg/g) |
ICP-OES (AOAC 984.27 mod, 927.02 mod, 985.01 mod, 965.17 mod) |
|
L-Threonate |
82 to 91% |
HPLC |
|
Ethanol residues |
≤ 5000 ppm |
USP 467 |
|
pH |
5.8 – 7.0 |
USP (1% in H2O) |
|
Arsenic |
≤ 1 ppm |
ICP-MS USP <730> |
|
Mercury |
≤ 0.1 ppm |
ICP-MS USP <730> |
|
Lead |
≤ 0.5 ppm |
ICP-MS USP <730> |
|
Cadmium |
≤ 0.2 ppm |
ICP-MS USP <730> |
|
Total plate count |
≤ 3000 CFU/g |
USP <2021> |
|
Yeast and mould |
≤ 100 CFU/g |
USP <2021> |
|
E. coli |
Absent in 1g |
USP <2022> |
|
Salmonella |
Absent in 25g |
USP <2022> |
NLT = not less than; NMT = not more than; ND = not determined; ppm = parts per million; CFU = colony forming units
26. Oxalic acid, which is used as a reagent in this production process, was detected at 0.155% w/w in magnesium L-threonate monohydrate.
27. Oxalic acid is an antinutrient which can adversely affect the body’s ability to handle essential minerals such as iron, magnesium and especially calcium. In particular, calcium oxalate can form and precipitate in the kidneys and bladder leading to the development of kidney and bladder stones. The addition of a maximum specified limit for oxalic acid/oxalate content in the novel food is recommended as a risk management measure.
28. The information provided is sufficient for the specification of magnesium L-threonate monohydrate, and appropriately characterises the novel food seeking authorisation.
2.6 History of Use
29. Magnesium is recognised as an essential mineral which has a key role in many physiological processes (EFSA, 2016). L-threonate is a recognised metabolite of ascorbic acid and is also an endogenous compound in the body (EFSA, 2008).
30. The novel food has no history of use in the EU. However, calcium L-threonate, the production intermediate of magnesium L-threonate monohydrate, is authorised as a source of calcium (EFSA, 2008).
31. Magnesium L-threonate monohydrate is recognised as a GRAS ingredient in the USA and is also approved in Canada with a health claim as a dietary supplement.
32. The history of use does not indicate any further areas for evaluation.
2.7 Proposed Use and Anticipated Intake
33. Magnesium L-threonate monohydrate is intended to be used by the adults only to replace other sources of magnesium in food supplements. The novel food is not intended to be used in addition to other sources of magnesium.
34. The maximum dose of the novel food is 3,000 mg/day, which will provide up to 249 mg/day of magnesium and up to 2,730 mg/day of L-threonate.
35. The tolerable upper intake level for magnesium from readily dissociable magnesium salts (e.g., chloride, sulphate, aspartate, lactate), used in food supplements or added to foods, is 250 mg/day (EFSA, 2015).
36. Dietary surveys report that the estimated mean and high-level intakes (97.5th percentile) for magnesium from dietary sources range from 208 – 327 mg/day and 350 – 628 mg/day, respectively (EFSA, 2006). By comparison, the adequate intake (footnote) values for magnesium in men and women are reported as 350 and 300 mg/day, respectively (EFSA, 2015).
37. Magnesium is typically bound or chelated (e.g. chlorophyll; phosphate; phytate) in food and beverages. Consequently, consumer intake levels from dietary sources of magnesium are expected to be limited due to the low bioavailability of the mineral (EFSA, 2006).
38. L-threonate is not typically consumed as a food. In the previous safety assessment of calcium L-threonate as a source of calcium (EFSA, 2008), the margin of safety was considered sufficiently large given that L-threonate is an endogenous compound in the body. Since the levels of L-threonate in the novel food are comparable, this is not expected to be a cause for concern for consumers of magnesium L-threonate monohydrate. However, the consumption of calcium and magnesium L-threonate on the same day should be contraindicated.
2.8 Absorption, Distribution, Metabolism and Excretion (ADME)
39. Active transport systems tightly control the amount of magnesium that crosses from the digestive tract into the bloodstream. Therefore, the absorption of magnesium from the novel food requires magnesium L-threonate monohydrate to be a dissociable salt under physiological conditions in the gastrointestinal tract. Patel, (2020) [unpublished paper] demonstrated that magnesium L-threonate monohydrate can dissociate under acidic conditions (pH 2) to magnesium ions and L-threonate ions, albeit not under neutral conditions.
40. The absorption of magnesium from the novel food has been confirmed by Liu et al. (2016). A prospective, randomised, double-blind, placebo-controlled, parallel-group clinical trial examining serum magnesium levels over a 12-week period following supplementation with magnesium-L-threonate monohydrate reported that magnesium is absorbed following the ingestion of novel food in humans.
41. A study in Sprague-Dawley rats established that approximately 60% of the magnesium L-threonate dose is absorbed compared to approximately 40% for magnesium chloride, magnesium citrate or magnesium gluconate (Slutsky et al., 2010). A more recent study in rats confirmed that magnesium L-threonate has a higher absorption than magnesium chloride and magnesium sulphate (Sadir et al., 2019).
42. The ADME of magnesium L-threonate monohydrate does not indicate any further areas for concern.
2.9 Nutritional information
43. The novel food is magnesium L-threonate monohydrate which is specified at 82 – 91% L-threonate and 7.2 – 8.3% magnesium by weight.
44. The maximum dose level for the novel food is comparable to the upper tolerable daily intake value for magnesium. Reports have suggested that magnesium is “the natural calcium antagonist” which can inhibit calcium enteral absorption (EFSA, 2006). Spencer et al. (1994) reported that magnesium intakes of 576 mg/day had no impact on the calcium levels in human volunteers. The Expert Group on Vitamins and Minerals stated that based on a majority of studies, 400 mg/day of magnesium supplements would not be expected to result in any significant adverse effects (EVM, 2003). Therefore, the evidence that magnesium has a negative influence on the serum calcium levels under physiological conditions was considered to be weak (EFSA, 2006).
45. Oxalic acid is a reactant in the novel food production process. Analysis from one batch of the novel food demonstrated that residues of this substance were present at 0.155% w/w. Given that the maximum intake for magnesium L-threonate monohydrate is 3,000 mg/day, this would provide a daily dose of oxalic acid equivalent to 4.65 mg/day.
46. Holmes and Kennedy (2000) reported that oxalic acid exposure levels from a small sample of consumers ranged from 44 to 352 mg/day (mean intake of 152 mg/day). Noonan and Savage (1999) calculated mean exposure levels to oxalic acid in the English diet as 70 – 150 mg/day.
47. Based on this information, the consumption of the novel food as a source of magnesium is not expected to be nutritionally disadvantageous for consumers at the maximum use levels specified in this application.
2.10 Toxicological information
48. Toxicological studies were performed with magnesium L-threonate monohydrate to support the safety assessment of the novel food. The respective study reports are unpublished and claimed as proprietary data. They were reviewed by the ACNFP and considered essential in the assessment of the safety of the novel food.
2.10.1 Genotoxicity
49. In vitro genotoxicity testing of magnesium L-threonate monohydrate was conducted under Good Laboratory Practice (GLP) conditions and according to the OECD guidelines: in vitro bacterial reverse mutation test (OECD TG 471) and in vivo mammalian erythrocyte micronucleus test (OECD TG 474). This is not the approach recommended by the UK Committee on Mutagenicity or in the guidance on the preparation and submission of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283.
50. The Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA, 2011) recommends two in vitro tests as the first step in testing for genotoxicity: in vitro bacterial reverse mutation test (OECD TG 471) and in vitro mammalian cell micronucleus test (OECD TG 487). This approach addresses the key endpoints for adequately assessing genotoxicity with the minimum number of tests and avoiding unnecessary animal tests.
51. The in vivo mammalian erythrocyte micronucleus test assesses both structural and numerical chromosomal aberrations and is an appropriate follow-up test for in vitro clastogens and aneugens. Therefore, this in vivo test, which is valid because the ADME data confirms the bone marrow cells are exposed to the novel food, along with the in vitro bacterial reverse mutation test, provide assurance that the genotoxicity of the novel food has been adequately assessed.
52. The in vitro bacterial reverse mutation test (Harke, 2020 [unpublished]) demonstrated that magnesium L-threonate monohydrate is non-mutagenic, in the absence or presence of metabolic activation.
53. The in vivo mammalian erythrocyte micronucleus test (Sai Kumar, 2021 [unpublished]) demonstrated that magnesium L-threonate monohydrate is non-clastogenic and non-aneugenic.
54. The results from these in vitro and in vivo studies support the conclusion that the novel food is not genotoxic.
2.10.2 Sub-chronic toxicity
55. A Repeated Dose 90-Day Oral Toxicity Study in Rodents (Saravanan, 2021 [unpublished]) was conducted under GLP conditions according to OECD TG 408 guidelines as recommended by the Guidance on the preparation and submission of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283. The aim of the study was to identify any adverse effects following the consumption of magnesium-L-threonate monohydrate.
56. In this 90-day feeding study, each group consisted of 10 female and 10 male Sprague Dawley rats which were fed 0 (control – vehicle only [water]), 500, 1,000 or 2,000 mg/kg BW/day of magnesium-L-threonate monohydrate by oral gavage. Additionally, satellite groups consisting of 6 female rats and 6 male rats for the control and high dose treatment were used as recovery groups.
57. No deaths, test item-related clinical abnormalities, ocular changes, or differences in food consumption and bodyweight between test groups were reported. In addition, there were no statistically significant dose dependent changes in haematology, clinical chemistry, serum hormone levels, urinalysis, or organ weights.
58. No dose related abnormalities were noted during the necropsy or histopathological evaluation. Therefore, the no observable adverse effect level (NOAEL) for magnesium-L-threonate monohydrate was considered to be the highest dose tested of 2,000 mg/kg BW/day.
2.10.3 Human studies
59. A prospective, randomized, double-blind, placebo-controlled, parallel-group clinical trial following fifty male and female adults (aged 50 – 70 years old) for 12 weeks was conducted (Liu et al., 2016). Doses of 1.5 g/day were given to the participants who weighed under 70kg, and doses of 2.0 g/day were given to those who weighed 70 – 100kg. No adverse effect related safety concerns were reported during the trial.
2.11 Allergenicity
60. Magnesium L-threonate monohydrate is a chemically purified substance which is not expected to produce an IgE-mediated allergenic response in consumers.
3. Discussion
61. The novel food is magnesium L-threonate monohydrate, which consists of 7.2 – 8.3% w/w magnesium, 82 – 91% w/w L-threonate and ≤ 5% w/w water.
62. Magnesium L-threonate monohydrate is manufactured by a two-step chemical synthetic process to yield a water soluble white to off-white powder with a purity of 98 – 102%.
63. Magnesium L-threonate monohydrate is intended to be used as a source of magnesium and is proposed to replace other sources of magnesium in food supplements only. The novel food is not intended to be used in addition to other sources of magnesium. The maximum dose of magnesium L-threonate monohydrate is 3,000 mg/day which provides up to 249 mg/day of magnesium and up to 2,730 mg/day of L-threonate.
64. The NOAEL for the magnesium L-threonate monohydrate in the sub-chronic rat study is 2,000 mg/kg BW/day.
65. The exposure to magnesium L-threonate monohydrate on a body weight basis for consumers over 18 years is 39.5 – 42.1 mg/kg BW/day. These values were derived from the maximum dose of 3,000 mg/day and the default bodyweights for the adult, the elderly, and the very elderly sub-populations of 73.9 kg, 76.0 kg and 71.2 kg, respectively (EFSA, 2012).
66. The Margin of Safety (MoS) for the novel food was calculated by comparing the NOAEL for the magnesium L-threonate monohydrate (2,000 mg/kg BW/day) with the exposure of magnesium L-threonate monohydrate on a body weight basis (39.5 – 42.1 mg/kg BW/day). The resulting MoS for magnesium L-threonate monohydrate ranges from 48 to 51 in these sub-populations.
67. The estimated mean and high-level intakes (97.5th percentile) for magnesium from dietary sources range from 208 – 327 mg/day and 350 – 628 mg/day, respectively (EFSA, 2006). However, the magnesium in foods and beverages is typically bound or chelated, which accounts for the low bioavailability of this mineral from dietary sources.
68. The maximum daily dose of the novel food could provide up to 249 mg/day of magnesium. Animal studies indicate that the absorption of magnesium from the novel food, although reportedly higher compared to other dissociable magnesium salts, is not 100% of the dose. Therefore, consumer exposure levels to magnesium from the novel food are expected to be lower than the tolerable upper limit for magnesium of 250 mg/day.
4. Conclusion
69. The ACNFP have undertaken the assessment of magnesium L-threonate monohydrate and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake level and the proposed use in food supplements was not considered to be nutritionally disadvantageous.
70. These conclusions were based on the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
- bioavailability study (Zhao et al. [unpublished]); in vitro bacterial reverse mutation test (Harke, 2020 [unpublished]), in vivo micronucleus test (Sai Kumar, 2021 [unpublished]); 90-day repeat dose feeding study with the novel food (Saravanan, 2021 [unpublished]) and double-blind human clinical trial (Krieger et al., 2013 [unpublished]
Acknowledgements
With thanks to the members of the ACNFP during the course of the assessment who were; Dr Camilla Alexander White, Dr Anton Alldrick, Alison Austin, Dr Mark Berry, Professor Dimitris Charalampopoulos, Professor Susan Fairweather-Tait, Professor Paul Fraser, Dr Hamid Ghoddusi, Dr Andy Greenfield, Professor Wendy Harwood, Professor Huw Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry McArdle, Rebecca McKenzie, Professor Clare Mills, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, Professor Bruce Whitelaw, Dr Cathrina Edwards, Professor George Bassel, Dr Kimon-Andreas Karatzas, Dr Christine Bosch and Dr Antonio Peña-Fernández.
References
EFSA, 2015. NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific Opinion on Dietary Reference Values for magnesium. EFSA Journal 13(7):4186 [63 pp.] https://doi.org/10.2903/j.efsa.2015.4186
EFSA, 2021. NDA Panel (Panel on Dietetic Products, Nutrition and Allergies), 2021. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283 (Revision 1). Published 26 March. https://doi.org/10.2903/j.efsa.2011.2170
EFSA, 2006. Scientific Committee on Food, NDA Panel (Panel on Dietetic Products, Nutrition and Allergies). Tolerable Upper Intake Levels for Vitamins and Minerals. Published February [482 pp.]. ISBN: 92-9199-014-0.
www.efsa.europa.eu/sites/default/files/assets/ndatolerableuil.pdf
EFSA, 2008. Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to food (ANS) on a request from the Commission on calcium L-threonate as a source for calcium added for nutritional purposes in food supplements. EFSA Journal 866, 1-20. https://doi.org/10.2903/j.efsa.2008.866
EFSA, 2011.Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 9(9):2379 [69 pp.]
https://doi.org/10.2903/j.efsa.2011.2379
EFSA, 2012. Scientific Committee Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 10(3):2579. [32 pp.]
https://doi:10.2903/j.efsa.2012.2579.
EFSA, 2021. Scientific Committee on Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 19(8):6769 [48 pp.]
https://doi.org/10.2903/j.efsa.2021.6769
European Commission, 2011. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004
https://doi.org/10.1017/cbo9780511664885.043
Expert Group on Vitamins and Minerals, 2003. Safe Upper Levels for Vitamins and Minerals. Published May [360 pp.].
https://doi.org/10.1046/j.1467-3010.2003.00344.x
Harke AN [unpublished]. Dated 26 November 2020. Vipragen Biosciences Private Limited, Karnataka, India. Study Title: Bacterial Reversion Mutation (Ames) Assay of Magtein® using Salmonella typhimurium tester strains. (Study number VBPL-005/2020)
Holmes RP and Kennedy M, 2000. Estimation of the oxalate content of foods and daily oxalate intake. Kidney International. April; 57(4):1662-7
https://doi.org/10.1046/j.1523-1755.2000.00010.x
Liu G, Weinger JG, Lu ZL, Xue F, Sadeghpour S, 2016. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Alzheimer’s Disease 49(4) pp 971-990. https://doi.org/10.3233/jad-150538
Noonan SC and Savage GP (1999). Oxalate content of foods and its effects on humans. Asia Pacific Journal of Clinical Nutrition. March; 8(1): 64-74.
https://doi.org/10.1046/j.1440-6047.1999.00038.x
OECD, 1997. Bacterial reverse mutation test. In OECD guidelines for the testing of chemicals. OECD guideline No 471 (updated & adopted: 21 July 1997). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264071247-en
OECD, 1998. OECD principles of good laboratory practice. Series on principles of good laboratory practice and compliance monitoring, No. 1 (ENV/MC/CHEM(98) 17). Paris, France: Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Chemicals Group and Management Committee.
https://doi.org/10.1787/9789264078536-en
OECD, 2016a. Mammalian in vivo micronucleus test. In OECD guidelines for the testing of chemicals. OECD guideline No 474 (updated & adopted: 29 July 2016). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264264762-en
OECD, 2016b. In vitro mammalian cell micronucleus test. In OECD guidelines for the testing of chemicals. OECD guideline No 487 (updated & adopted: 29 July 2016). Paris, France: Organisation for Economic Co-operation and Development (OECD).
https://doi.org/10.1787/9789264264861-en
OECD, 2018. Repeated dose 90-day oral toxicity study in rodents. In OECD guidelines for the testing of chemicals. OECD guideline No 408 (updated and adopted 27 June 2018). Paris, France: Organisation for Economic Co-operation and Development (OECD). https://doi.org/10.1787/9789264070707-en
Patel B [unpublished]. Dated 20 April 2020. Chromak Research, Inc., New Jersey, USA. Study Title: Demonstration of Dissociation of Magnesium-L-Threonate (Magtein®) at Various pH by HPLC-UV, ICP-MS and Identification of the Starting Material as Magnesium-L-Threonate (Magtein) by Tandem Mass Spectrometry. CRI (Study number CR0420003).
Sadir S, Tabassum S, Emad S, Liaquat L, Batool Z, Madiha S, Shehzad S, Sajid I, Haider S, 2019. Neurobehavioral and biochemical effects of magnesium chloride (MgCl2), magnesium sulphate (MgSO4) and magnesium-L-threonate (MgT) supplementation in rats: A dose dependent comparative study. Pakistan Journal of Pharmaceutical Sciences. Jan; 32(1) Supplementary pp 277-283.
https://doi.org/10.17582/journal.pjz/2018.50.4.1245.1256
Sai Kumar M [unpublished]. Dated 23 February 2021. Vipragen Biosciences Private Limited, Karnataka, India. Study Title: In Vivo Micronucleus Test of Magtein® in Swiss Albino Mice (Study number VBPL-006/2020)
Saravanan M [unpublished]. Dated 22 February 2021. Vipragen Biosciences Private Limited, Karnataka, India. Study Title: 90-Day Repeated Oral (Gavage) Toxicity Study of Magtein® in Sprague Dawley Rats with a 28-Day Recovery Period (Study number VBPL-007/2020)
Slutsky I, Abumaria N, Wu LJ, Huang C, Zhang L, Li B, Zhao X, Govindarajan A, Zhao MG, Zhuo M, Tonegawa S, Liu G, 2010. Enhancement of learning and memory by elevating brain magnesium. Neuron. Jan 28;65(2):165-77.
https://doi.org/10.1016/j.neuron.2009.12.026
Spencer H, Fuller H, Norris C, Williams D, 1994 Effect of magnesium on the intestinal absorption of calcium in man. Journal of the American College of Nutrition. October;13(5) pp 485-92. https://doi.org/10.1080/07315724.1994.10718439